- Title
-
NEDD8 enhances Hippo signaling by mediating YAP1 neddylation
- Authors
- Chen, M., Liu, Y., Zuo, M., Guo, C., Du, Y., Xu, H., Liu, B., Li, M., Xiao, W., Yu, G.
- Source
- Full text @ J. Biol. Chem.
|
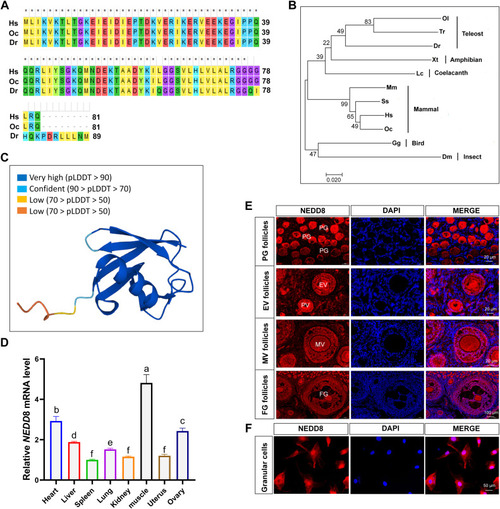
Phylogenetic analysis of NEDD8 proteins and the expression profile of rabbit NEDD8 in different tissues. A, alignment of NEDD8 amino acid sequences from Homo sapiens (Hs), Oryctolagus cuniculus (Oc), and Danio rerio (Dr). B, phylogenetic tree of NEDD8 proteins in 11 species constructed using the neighbor-joining method. Homo sapiens (Hs), Gene ID: 4738; Oryctolagus cuniculus (Oc), Gene ID: 100009008; Mus musculus (Ms), Gene ID: 18002; Sus scrofa (Ss), Gene ID: 100520086; Gallus gallus (Gg), Gene ID: 100858776; Latimeria chalumnae (Lc), Gene ID: 102345978; Xenopus tropicalis (Xt), Gene ID: 549727; Danio rerio (Dr), Gene ID: 368667; Oryzias latipes (Ol), Gene ID: 101172934; Takifugu rubripes (Tr), Gene ID: 101077379; Drosophila melanogaster (Dm), Gene ID: 35151. C, predicted protein structure of rabbit NEDD8 by UniProtKB in the Ensemble database ( https://www.uniprot.org/uniprotkb/Q4PLJ0/entry ). D, mRNA expression levels of NEDD8 in various tissues of adult rabbits. Data are expressed as mean ± SD, n = 3 experiments. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. E, immunofluorescence staining of NEDD8 in follicles at different developmental stages in rabbit ovaries. The scale bar represents 20 μm and 100 μm, respectively. F, confocal microscopy image of endogenous NEDD8 localization in rabbit GCs. NEDD8 is stained red and nuclei are stained blue with DAPI. The scale bar represents 50 μm. DAPI, 4′, 6-diamidino-2-phenylindole; EV, early vitellogenic stage; FG, full-grown stage; GC, granulosa cell; MV, mid-vitellogenic stage; ns, not significant; PG, primary growth stage; PV, previtellogenic stage. |
|
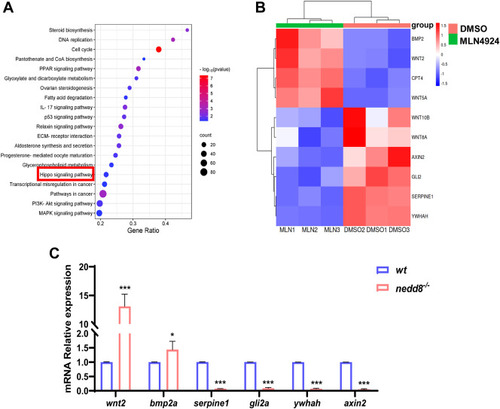
Bioinformatic analysis of differentially expressed genes in rabbit ovarian GCs treated with DMSO or MLN4924. A, representative KEGG enrichment results of differentially expressed genes between rabbit ovarian GCs of DMSO and MLN4924 treatment, respectively. The data point size indicates the number of enriched genes. | log2 FoldChange | >1.5 and p value <0.05 are set as screening criteria. B, expression heatmap of DEGs in the Hippo pathway. Red represents increased gene expression, while blue represents decreased gene expression. The color legend shows the FPKM values each represents. C, qPCR analysis of DEGs related to Hippo pathway in zebrafish. Data are expressed as mean ± SD, n = 3 experiments. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001, using unpaired Student’s t test. DEG, differentially expressed gene; DMSO, dimethyl sulfoxide; FPKM, fragments per kilobase million; GC, granulosa cell; KEGG, Kyoto encyclopedia of genes and genomes; ns, not significant; qPCR, quantitative real-time PCR. |
|
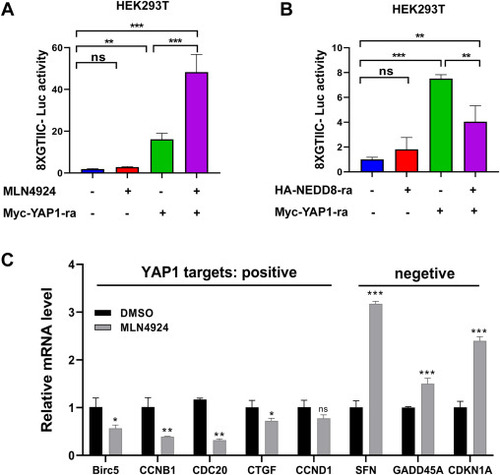
Neddylation enhances YAP-mediated signaling. A, 8xGTIIC-Luc reporter activity in Myc empty vector (200 ng) or Myc-YAP1 (200 ng) transfected HEK293T cells with or without HA-NEDD8 for 36 h. B, 8xGTIIC-Luc reporter activity in Myc empty vector (200 ng) or Myc-YAP1 (200 ng) transfected HEK293T cells with or without MLN4924 (1 μM) treatment for 24 h. C, quantitative real-time PCR (qPCR) analysis of genes either positively or negatively regulated by YAP1 in rabbit GCs treated with or without MLN4924 (1 μM) for 24 h. Four separate experiments were performed, with each measurement performed at least three times. Data are expressed as mean ± SD. (unpaired two-tailed Student's t test). ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. GC, granulosa cell; ns, not significant; YAP1, yes-associated protein 1. |
|
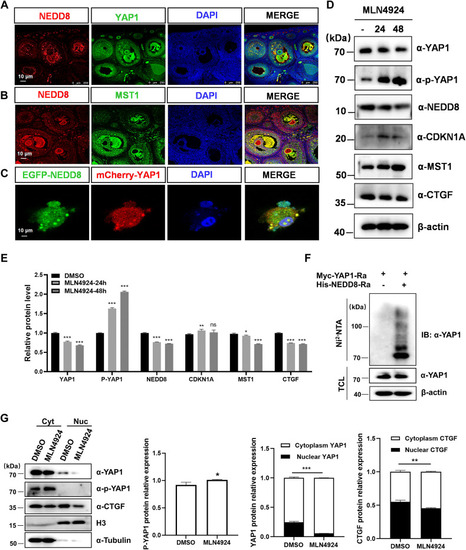
Neddylation of YAP1 promotes its nuclear translocation. A and B, immunofluorescence staining of endogenous NEDD8 and MST1 (A), NEDD8 and YAP1 (B) expression, and localization in the rabbit ovary. The nucleus was stained blue with DAPI (the scale bar represents 100 μm). C, confocal microscopy image of cotransfection with EGFP-NEDD8 and mCherry-YAP1 plasmids in H1299 cells, the nucleus was stained blue with DAPI (the scale bar represents 10 μm). D, Western blot analysis of the indicated protein levels of YAP1, p-YAP1, NEDD8, CDKN1A, MST1, and CTGF in rabbit GCs treated with or without MLN4924 (1 μM) for 0 to 48 h. E, quantification of the above proteins expression level. F, neddylation of YAP1 is detected by immunoprecipitation and Western blot in HEK293T cells in response to NEDD8 overexpression using Ni-NTA agarose beads. G, Western blot analysis and the quantification of the indicated protein in nuclear (Nuc) versus cytoplasmic (Cyt) fractionation from rabbit GCs with or without MLN4924 (1 μM) treatment for 24 h. Data are represented as means ± SD, n = 3 experiments, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. Cyt, cytoplasm; DAPI, 4′, 6-diamidino-2-phenylindole; GC, granulosa cell; IB, immunoblotting; IP, immunoprecipitation; ns, not significant; Nuc, nucleus; TCL, total cell lysate; YAP1, yes-associated protein 1. |
|
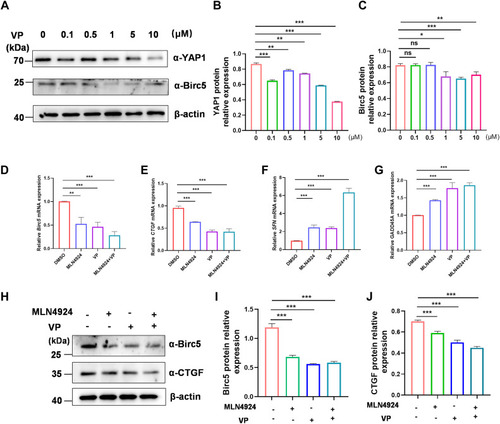
MLN4924 and VP work together to inhibit YAP1-targeted gene expression of GCs. A–C, GCs were treated with VP at various doses (0, 0.1, 0.5, 1, 5, 10 μM) for 24 h. Cell lysates were subjected to Western blotting, and the protein levels of YAP1 and Birc5 were quantified and normalized to that of β-actin. A, representative experiment from three separate experiments is shown. B, quantification of YAP1 protein expression level in VP-treated cells. C, quantification of Birc5 protein expression level in VP-treated cells. D–G, qPCR analysis of YAP1-activated target genes Birc5 (D) and CTGF (E) mRNA levels, and YAP1-inhibited target genes SFN (F) and GADD45A (G) mRNA levels in GCs treated with DMSO, ML4924 (1 μM), VP (5 μM), or ML4924 and VP together for 24 h. H–J, GCs were treated with DMSO, ML4924, VP, or ML4924 and VP together for 24 h. Cell lysates were used for Western blot, and CTGF and Birc5 protein levels were subsequently quantified and normalized to that of β-actin. A representative Western blot (H), the quantification of CTGF (I) and Birc5 (J) from three independent experiments. Data are presented as means ± SD, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. DMSO, dimethyl sulfoxide; GC, granulosa cell; ns, not significant; qPCR, quantitative real-time PCR; VP, verteporfin; YAP1, yes-associated protein 1. |
|
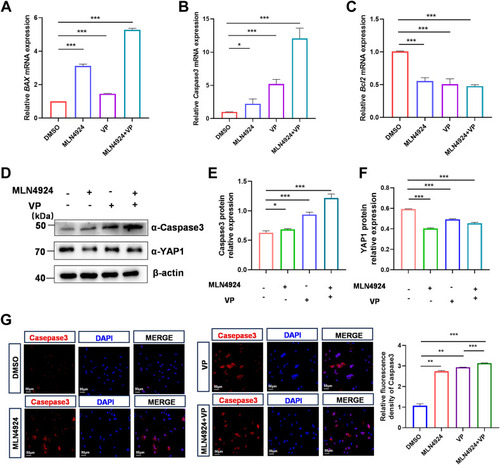
Effect of YAP1 suppression on MLN4924-induced apoptosis in rabbit GCs. A–C, qPCR analysis of proapoptotic marker genes Bax (A), Caspase3 (B), and antiapoptotic marker gene Bcl2 (C) mRNA levels in GCs treated with DMSO, MLN4924 (1 μM), VP (1 μM), or MLN4924 and VP together for 24 h. D–F, GCs were treated with DMSO, MLN4924 (1 μM), VP (5 μM), or MLN4924 and VP together for 24 h. Cell lysates were used for Western blot, and then YAP1 and Caspase3 protein levels were quantified and normalized to that of β-actin. A representative Western blot (D), the quantification of YAP1 (E) and Caspase3 (F) from three independent experiments. G, representative immunofluorescence staining for Caspase3 (red) after DMSO, MLN4924 (1 μM), VP (5 μM), or MLN4924 and VP together for 48 h. Quantification analysis of relative Caspase3 expression level (fluorescence intensity of Caspase3/DAPI) by Image J software. Data are presented as mean ± SD, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. DAPI, 4′, 6-diamidino-2-phenylindole; DMSO, dimethyl sulfoxide; GC, granulosa cell; ns, not significant; qPCR, quantitative real-time PCR; VP, verteporfin; YAP1, yes-associated protein 1. |
|
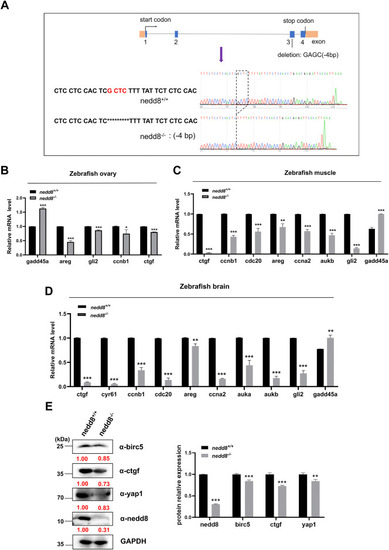
Disruption of nedd8 in zebrafish results in the inhibition of yap1 signaling. A, sequence information diagram of nedd8-null zebrafish. A 4-bp nucleotide deletion (5′-GAGC-3′) occurred in exon 3 of the nedd8 deletion mutant, resulting in a reading frame shift. B–D, qPCR analysis of yap1 target genes that are either positively or negatively regulated at the mRNA level in WT and nedd8-null zebrafish ovary (B), brain (C), and muscle (D). β-actin was used as an internal control. E, Western blot analysis of GAPDH, birc5, ctgf, yap1, and nedd8 protein levels in brain of nedd8+/+ and nedd8−/− zebrafish. GAPDH was used as an internal control. The red numbers represent the quantified results of the protein. Data are representative of three independent experiments (mean ± SD of three technical replicates). Unpaired two-tailed Student's t test was performed. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. ns, not significant; qPCR, quantitative real-time PCR; YAP1, yes-associated protein 1. |
|
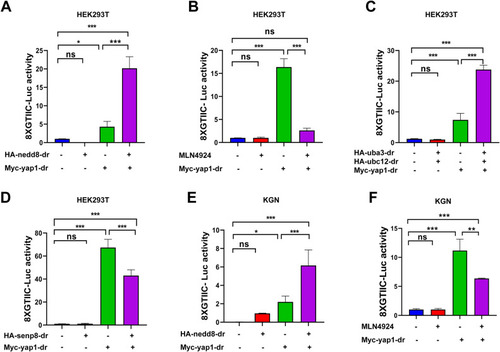
Zebrafish nedd8 enhances yap1 transcriptional activity. A, 8xGTIIC-Luc reporter activity in Myc empty vector (200 ng) or Myc-yap1 (200 ng) transfected HEK293T cells with or without HA-nedd8 for 36 h. B, 8xGTIIC-Luc reporter activity in Myc empty vector (200 ng) or Myc-yap1 (200 ng) transfected HEK293T cells with or without 1 μM MLN4924 treatment for 24 h. C, 8xGTIIC-Luc reporter activity in Myc empty vector (200 ng) or Myc-yap1 (200 ng) transfected HEK293T cells with or without HA-uba3 and HA-ubc12 for 36 h. D, 8xGTIIC-Luc reporter activity in Myc empty vector (200 ng) or Myc-yap1 (200 ng) transfected HEK293T cells with or without HA-senp8 for 36 h. E, 8xGTIIC-Luc reporter activity in Myc empty vector (200 ng) or Myc-yap1 (200 ng) transfected KGN cells with or without HA-nedd8 for 36 h. F, 8xGTIIC-Luc reporter activity in Myc empty vector (200 ng) or Myc-yap1 (200 ng)-transfected KGN cells with or without MLN4924 (1 μM) treatment for 24 h. Four separate experiments were performed, with each measurement performed at least three times. Data are expressed as mean ± SD. (unpaired two-tailed Student's t test). ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. ns, not significant; YAP1, yes-associated protein 1. |
|
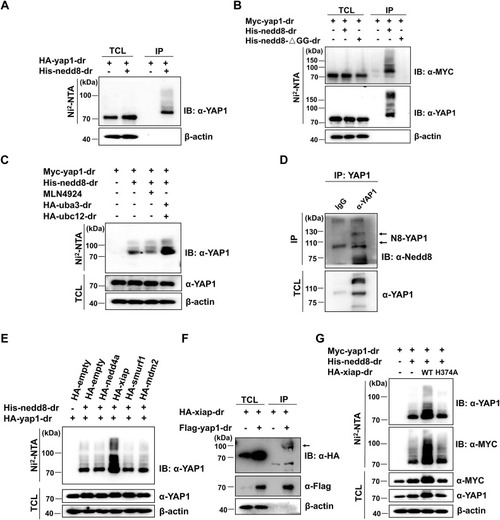
Xiap may act as an E3 ligase to catalyze Yap1 neddylation. A, neddylation of yap1 is detected by immunoprecipitation with Ni-NTA agarose and Western blot in HEK293T cells transfected with plasmids expressing HA-yap1 and PCI-His empty vector or His-nedd8 for 24 h. B, neddylation of yap1 is abolished by immunoprecipitation with Ni-NTA agarose and Western blot in HEK293T cells transfected with plasmids expressing Myc-yap1 and a conjugable mutant nedd8 (His-nedd8-ΔGG) for 24 h. C, neddylation of yap1 is detected by immunoprecipitation with Ni-NTA agarose and Western blot in HEK293T cells transfected with Myc-yap1, His-nedd8, and treated with MLN4924 (1 μM) for 12 h (third lane) or transfected with Myc-yap1, His-nedd8, HA-uba3, and HA-ubc12 (fourth lane). D, protein lysates from zebrafish brains were immunoprecipitated with mouse lgG (control) or anti-YAP1 antibody, respectively. Under partially denaturing conditions, coimmunoprecipitation was detected with anti-Nedd8 antibody. E, neddylation of yap1 is detected by immunoprecipitation with Ni-NTA agarose and Western blot in HEK293T cells transfected with HA-nedd4a, HA-xiap, HA-smurf1, and HA-mdm2 for 24 h. F, the interaction between yap1 and xiap is detected by immunoprecipitation with anti-FLAG agarose and Western blot in HEK293T cells transfected with the indicated plasmids for 24 h. G, neddylation of yap1 is detected by immunoprecipitation with Ni-NTA agarose and Western blot in HEK293T cells transfected with HA-xiap or xiap mutant (HA-xiap-H374A) for 24 h. IB, immunoblotting; IP, immunoprecipitation; TCL, total cell lysate; YAP1, yes-associated protein 1. |
|
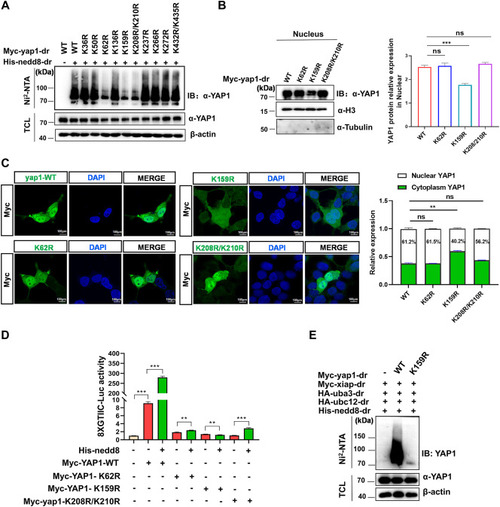
Xiap catalyzes Yap1 neddylation at lysine 159 (K159). A, neddylation of yap1 is detected by immunoprecipitation with Ni-NTA agarose and Western blot in HEK293T cells transfected with His-nedd8 and WT Myc-yap1 or one of the site-mutated yap1 constructs for 24 h. B, protein level of yap1 in nuclei is detected by Western blot analysis in HEK293T cells transfected with Myc-yap1-WT, K62R, K159R, or K208R/K210R. α-Tubulin and H3 are used as markers for the cytoplasmic and nuclear fractions, respectively. C, fluorescence of Myc is detected by confocal microscopy in HEK293T cells transfected with Myc-yap1 or yap1 site-mutant plasmids (K62R, K159R, K208R/K210R) for 24 h. The scale bar represents 100 μm. D, relative luciferase activity of the 8xGTIIC-Luc promoter in HEK293T cells expressing the indicated constructs together with or without His-nedd8. Four separate experiments were performed, with each measurement performed at least three times. E, neddylation of yap1 is detected by immunoprecipitation with Ni-NTA agarose and Western blot in HEK293T cells transfected with Myc-yap1-WT or Myc-yap1-K159R for 24 h. Data are expressed as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.IB, immunoblotting; IP, immunoprecipitation; TCL, total cell lysate; ns, not significant; YAP1, yes-associated protein 1. |