- Title
-
Tissue-specific and endogenous protein labeling with split fluorescent proteins
- Authors
- Ligunas, G.D., Paniagua, G., LaBelle, J., Ramos-Martinez, A., Shen, K., Gerlt, E.H., Aguilar, K., Nguyen, N., Materna, S.C., Woo, S.
- Source
- Full text @ Dev. Biol.
|
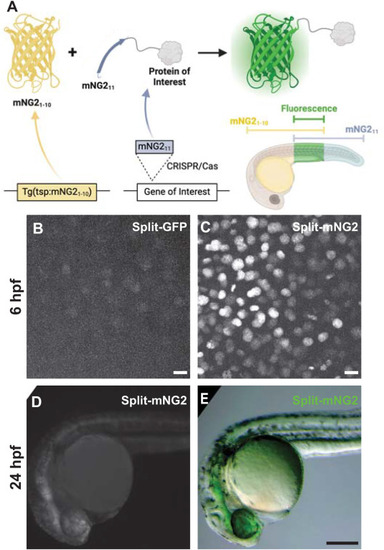
Split fluorescent protein fragments are functional in zebrafish embryos. A. Schematic illustrating our protein labeling strategy using a split fluorescent protein. Transgenic (Tg) mNG21-10 is expressed under the control of a tissue-specific promoter (tsp) while mNG211 is inserted into protein-coding genes by CRISPR/Cas-directed gene editing. Fluorescence (green) is only generated in tissues co-expressing mNG21-10 and the mNG211-tagged protein of interest. B–E. Embryos were injected with GFP1-10 and GFP11-H2B (split-GFP, B) or mNG21-10 and mNG211-H2B (split-mNG2, C–E) mRNAs then imaged at 6 h post-fertilization (hpf) on a confocal microscope (B, C) or at 24 hpf on a fluorescence stereomicroscope (D, E). Confocal images are displayed as maximum z-projections. Scale bars in B and C, 50 μm. Scale bar in E, 200 μm. |
|
Split fluorescent protein labeling can be spatially restricted by transgenic expression of mNG21-10. A–F. Transgenic embryos expressing mNG21-10 under control of the fezf2 (A–B), myl7 (C–D), or ubb (E–F) promoters and injected with mNG211-H2B mRNA. A′ shows the boxed region in A with brightness rescaled to demonstrate fluorescence is localized to nuclei. G–L. Transgenic embryos expressing GFP under control of the fez1 (G–H), myl7 (I–J), or ubb (K–L) promoters. Images were acquired at 24 h post-fertilization. Fluorescence images are maximum projections of confocal z-stacks. Scale bars in B, F, H, and L, 200 μm. Scale bars in D, J, 50 μm. |
|
mNG211 tagging by CRISPR/Cas-directed gene editing. A. Schematic of CRISPR/Cas-directed mNG211 insertion into target genes. Purple, endogenous exon sequence. Green, mNG211. Yellow, linker (LK). ATG, start codon. Arrows denote primers used in B. B. mNG211 insertion was assessed by PCR. The primers used correspond to the arrows shown in A. bp, base pairs. C. Amino acid sequences of wild-type, predicted mNG211 fusions, and recovered alleles for Tubb4b and Krt8. Mismatches between the predicted and recovered sequences are highlighted in red. Asterisks, stop codons. D–I. Representative images of mNG211-tubb4b (D–F) and krt8-mNG211 (G–I) embryos injected with mNG21-10 mRNA (D–E, G–H) or uninjected (F, I). Maximum projections of confocal z-stacks. Images were acquired at 24 h post-fertilization. Images in F and I have been overexposed to emphasize lack of fluorescence. Scale bars, 50 μm. |
|
Combinatorial expression of tissue-specific mNG21-10 and mNG211-tagged proteins. A. Schematic of crossing strategy. tsp, tissue-specific promoter. poi, protein of interest. B-D. Representative images of embryos obtained from crossing mNG211-tubb4b and ubb:mNG21-10 (B–B′), fez2f:mNG21-10 (C–C′), or myl7:mNG21-10 (D). B′ and C′ show boxed regions in B and C, respectively. E-H. Representative images of embryos obtained by crossing krt8-mNG211 to ubb:mNG21-10 (E–F), fez2f:mNG21-10 (G), or myl7:mNG21-10 (H). E′ shows boxed region in E. Maximum projections of confocal z-stacks. Images were acquired at 24 h post-fertilization (hpf) unless otherwise noted. Image in D has been overexposed to emphasize lack of fluorescence. Autofluorescent speckles (yolk, pigment cells, and debris) are colored blue for display purposes. Scale bars, 50 μm. |
|
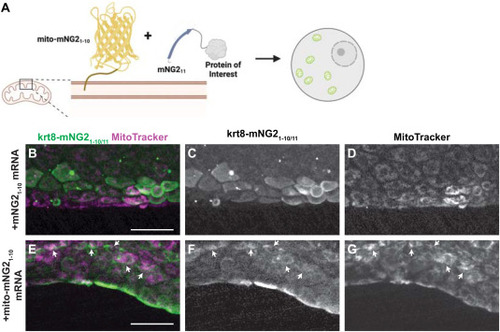
Directing protein localization with split-mNG2. A. Schematic illustrating use of the split-mNG2 system to sequester proteins of interest on mitochondria. B–G. Representative images of krt8-mNG211 embryos injected with mNG21-10 (B–D) or mito-mNG21-10 (E–G) mRNA and stained with MitoTracker dye to label mitochondria. Maximum projections of confocal z-stacks. Arrows indicate colocalization between split-mNG2 and MitoTracker fluorescence. Images were acquired from the tail fin epidermis at 48 h post-fertilization (hpf). Scale bars, 50 μm. |
Reprinted from Developmental Biology, 514, Ligunas, G.D., Paniagua, G., LaBelle, J., Ramos-Martinez, A., Shen, K., Gerlt, E.H., Aguilar, K., Nguyen, N., Materna, S.C., Woo, S., Tissue-specific and endogenous protein labeling with split fluorescent proteins, 109-116, Copyright (2024) with permission from Elsevier. Full text @ Dev. Biol.