- Title
-
Zebrafish as a Human Muscle Model for Studying Age-Dependent Sarcopenia and Frailty
- Authors
- Aranda-Martínez, P., Sayed, R.K.A., Fernández-Martínez, J., Ramírez-Casas, Y., Yang, Y., Escames, G., Acuña-Castroviejo, D.
- Source
- Full text @ Int. J. Mol. Sci.
|
Increase in frailty index with age. ( |
|
Histological and morphometric changes of zebrafish skeletal muscle. ( |
|
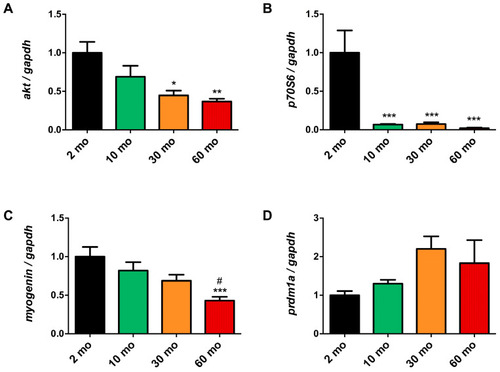
Disruption of muscle growth and differentiation pathways during aging. ( |
|
Changes in muscle ultrastructure and mitochondria. ( |
|
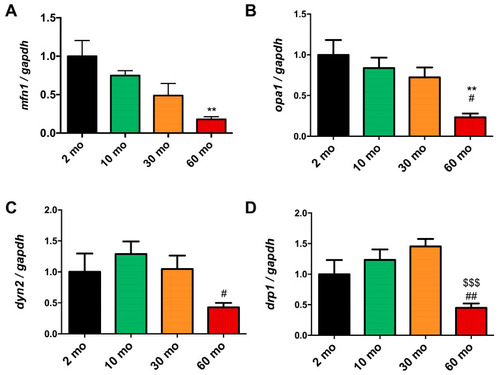
Reduction in mitochondrial dynamics in old fish. ( |
|
Loss of mitochondria with age. ( |