- Title
-
Dynamics of collagen oxidation and cross linking in regenerating and irreversibly infarcted myocardium
- Authors
- Akam-Baxter, E.A., Bergemann, D., Ridley, S.J., To, S., Andrea, B., Moon, B., Ma, H., Zhou, Y., Aguirre, A., Caravan, P., Gonzalez-Rosa, J.M., Sosnovik, D.E.
- Source
- Full text @ Nat. Commun.
|
Reactivity of a library of aldehyde-binding fluorescent probes. |
|
TMR-O shows superior selective binding to protein and tissue aldehydes. Created in part with Biorender.com. |
|
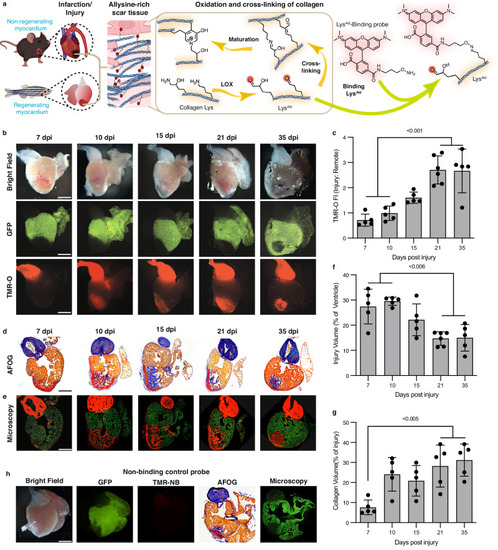
Collagen oxidation and cross-linking in the injured zebrafish heart. Created in part with Biorender.com. |
|
Collagen oxidation and cross-linking in the infarcted mouse heart. |
|
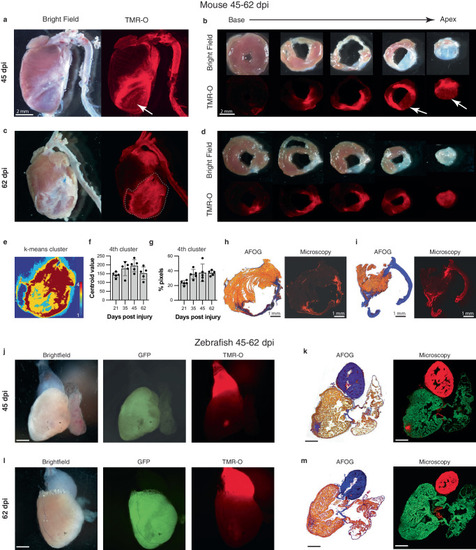
Dynamic deposition and oxidation of new collagen in chronic infarcts. |
|
Lysine aldehyde cross-links mature into degradation-resistant final products in infarcted mouse hearts but not in zebrafish hearts. Created in part with Biorender.com. |
|
Hydroxylation of lysine in newly deposited collagen differs markedly in infarcted murine and zebrafish hearts. HPLC traces of the amino acids in infarcted zebrafish ( |