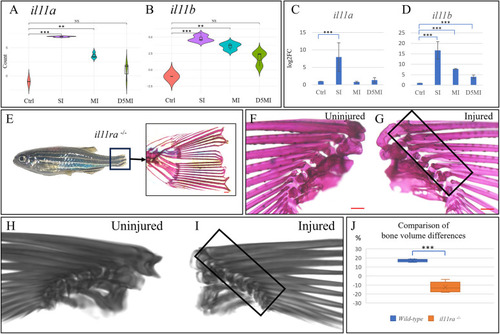
Differential expression of genes encoding Il-11, and absent heterotopic ossification in il11ra−/− mutants. (A,B) Violin plots depicting the differential expression of il11a and il11b as per RNA sequencing. The y-axis represents log2(normalised count). Control, no injury; SI, 24 h after single injury; MI, 24 h after multiple injuries; D5MI, 5 days after multiple injuries. n=9 fish per condition; n=3 fish per biological replicate. (C,D) qRT-PCR validation of the differential expression of il11a and il11b. n=9 fish per condition; n=3 fish per biological replicate. (E) il11ra−/− mutant zebrafish demonstrating impaired tail fin regeneration (boxed area) at 2 weeks following injury. The zoomed-in image shows an Alizarin Red-stained section of a non-regenerated tail fin. (F,G) Uninjured left and injured right pectoral fins of an il11ra−/− mutant zebrafish visualised 1 month post injury. (G) Medial view of the injured right pectoral fin showing no signs of heterotopic bone. (H,I) Three-dimensional reconstructed computed tomography scans. (I) Medial view of the injured right pectoral fin showing no heterotopic bone. (J) Box and whisker plot showing the comparison of bone volume differences (n=5 fish per group). The blue box represents the difference between bone volume of uninjured (control) and injured wild-type fins, expressed as percentages. The orange box represents the same for il11ra−/− mutants. Note the significant negative difference in il11ra−/− mutants. Boxes show the interquartile range, whiskers show the highest and lowest value, and the median is marked with a line. NS, not significant; **P<0.01; ***P<0.001 (Wilcoxon rank-sum test with Benjamini-Hochberg correction in A,B; two-tailed unpaired t-test in C,D,J). Scale bars: 200 µm (E-G).
|