- Title
-
High Oestrogen receptor alpha expression correlates with adverse prognosis and promotes metastasis in colorectal cancer
- Authors
- Topi, G., Satapathy, S.R., Ghatak, S., Hellman, K., Ek, F., Olsson, R., Ehrnström, R., Lydrup, M.L., Sjölander, A.
- Source
- Full text @ Cell Commun. Signal.
|
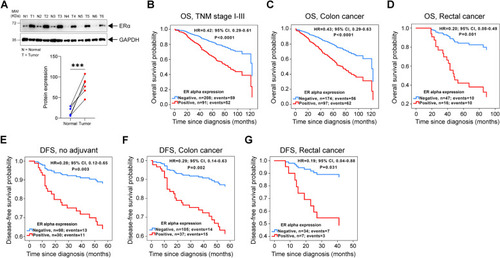
ERα expression and its prognostic association in colorectal cancer (CRC) patients. |
|
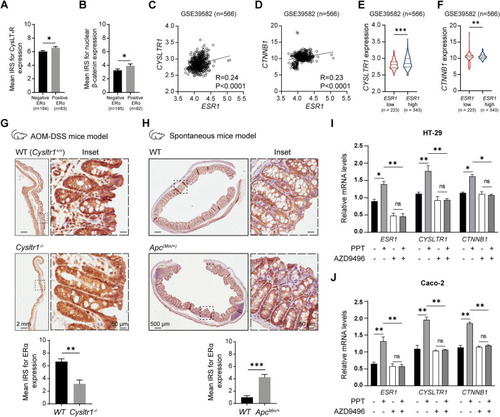
ERα expression positively correlates with tumour promoter expression in colon cancer. Mean immuno-reactive score (IRS) for ( |
|
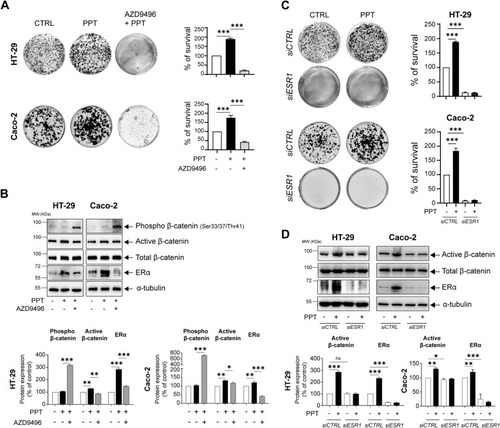
ERα activation in colon cancer cells promotes survival. |
|
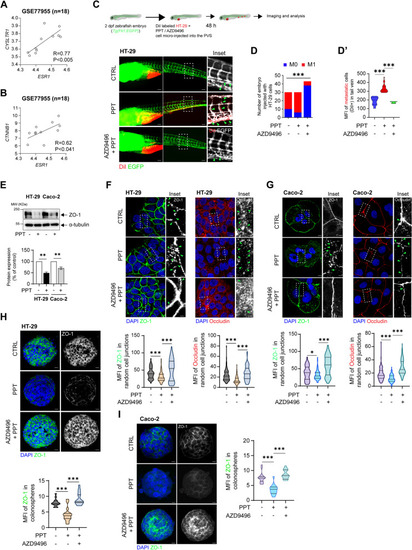
Activation of ERα promotes colon cancer cell metastasis. An external dataset composed of data for CC patients with liver metastasis (GSE77955, |
|
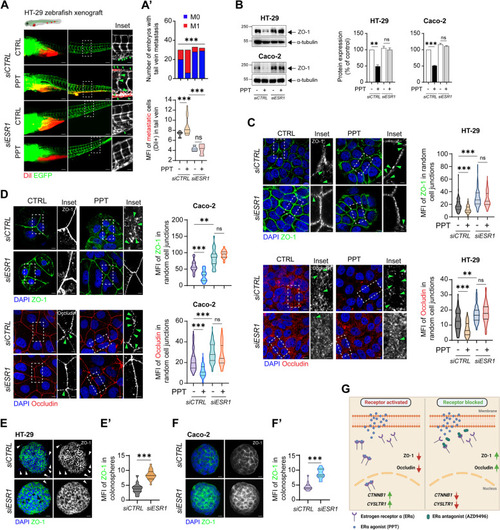
Functional absence of ERα inhibits colon cancer cell metastasis. DiI-labelled HT-29 cells transfected with either |