- Title
-
Engineered therapeutic antibodies with mannose 6-phosphate analogues as a tool to degrade extracellular proteins
- Authors
- Daurat, M., Gauthier, C., El Cheikh, K., Ali, L.M.A., Morère, E., Bettache, N., Gary-Bobo, M., Morère, A., Garcia, M., Maynadier, M., Basile, I.
- Source
- Full text @ Front Immunol
|
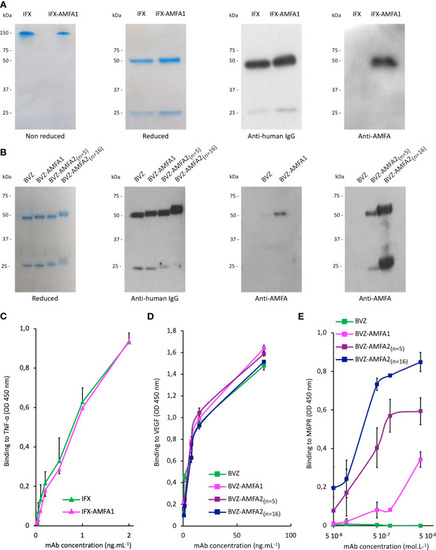
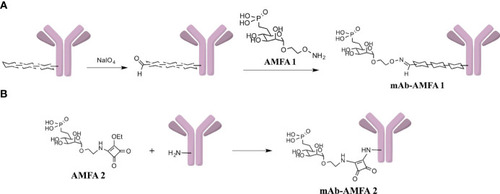
Characterization of Infliximab (IFX) and Bevacizumab (BVZ) before and after AMFA grafting. Analysis of IFX and IFX-AMFA1 |
|
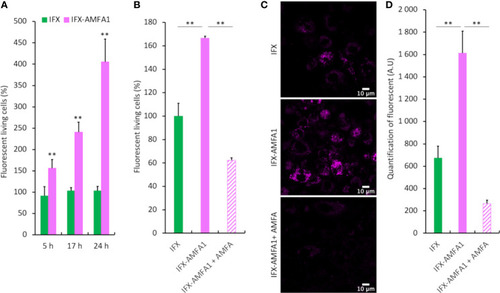
Cellular internalization of mAb and mAb-AMFA in cells and M6PR-dependency. After conjugation with AlexaFluor647® dye, 0.75 µg.mL-1 IFX or IFX-AMFA1 were incubated with Jurkat or macrophage-like cells in the presence of 0.188 µg.mL-1 TNF-α. Internalization of mAb quantified by fluorescence measurement by flow cytometry at different time points |
|
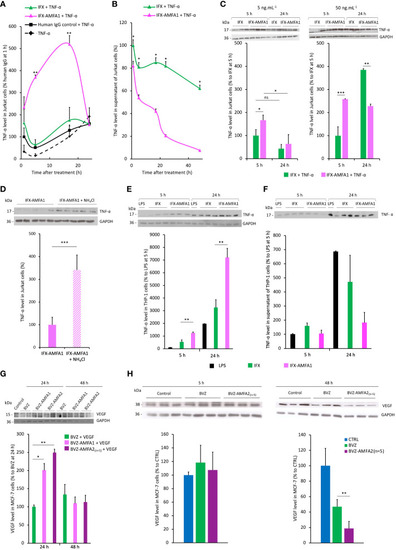
Internalization and degradation of TNF-α and VEGF in cell lines. Internalization in Jurkat cells treated with 20 ng.mL-1 IFX, IFX-AMFA1 or human IgG control in the presence of 5 ng.mL-1 TNF-α at different time points. Antigen levels in the cell lysates |
|
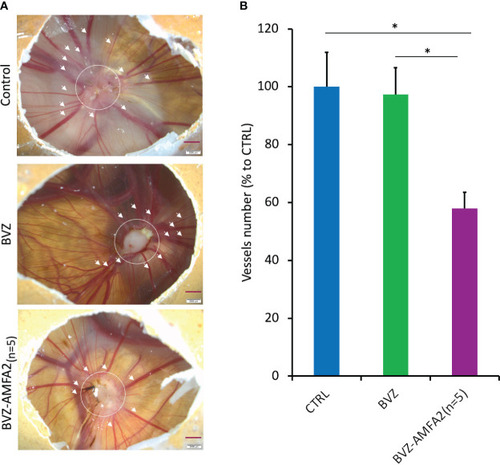
Neutralization of the cellular activities of antigens with mAb-AMFA. Murine fibroblasts L929 were treated with 5 ng.mL-1 TNF-α and increasing doses of IFX or IFX-AMFA1 |
|
|
|
|
|
|