- Title
-
Metformin Attenuates Neutrophil Recruitment through the H3K18 Lactylation/Reactive Oxygen Species Pathway in Zebrafish
- Authors
- Zhou, R., Ding, R.C., Yu, Q., Qiu, C.Z., Zhang, H.Y., Yin, Z.J., Ren, D.L.
- Source
- Full text @ Antioxidants (Basel)
|
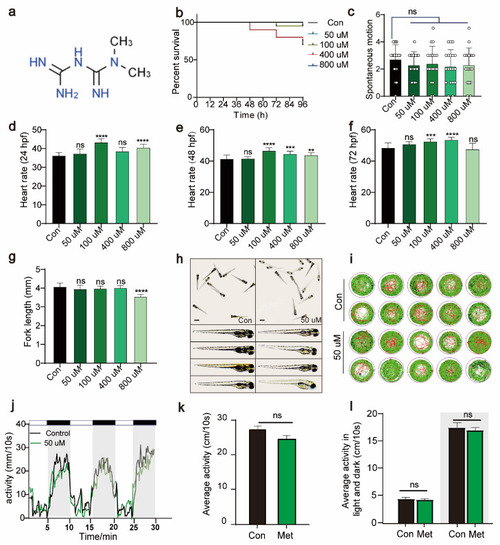
Metformin has no significant effect on the growth, development, or activity of zebrafish larvae. ( |
|
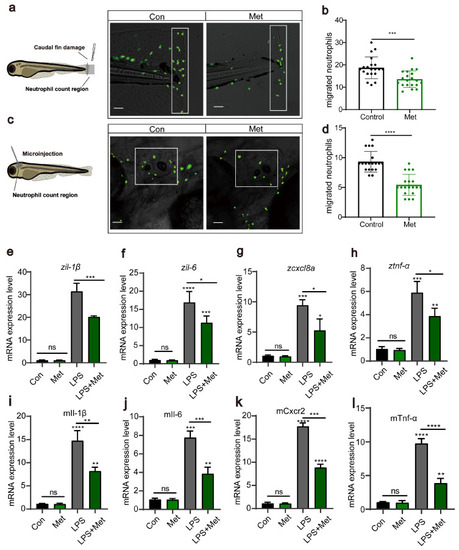
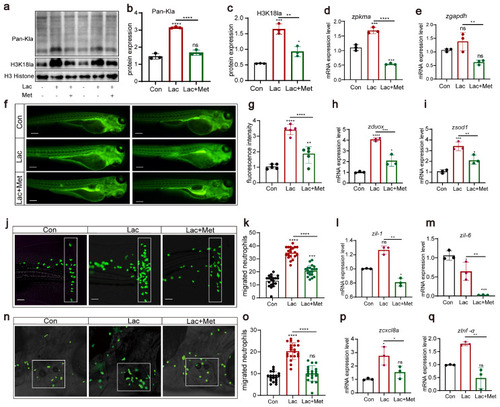
Metformin reduces the recruitment of neutrophils to inflammatory sites. ( |
|
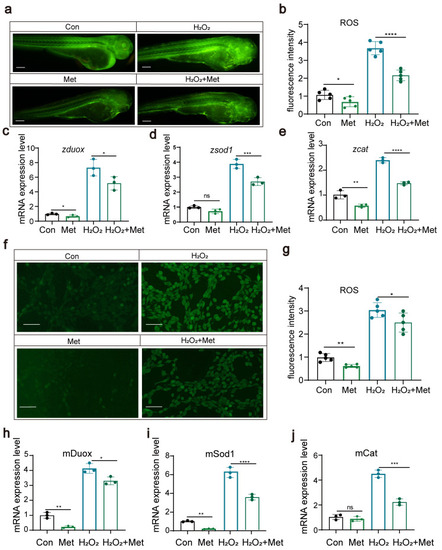
Metformin can reduce reactive oxygen species levels in zebrafish and cells ( |
|
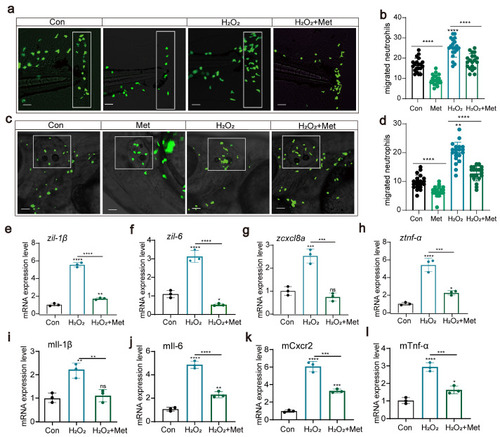
Reactive oxygen species can affect neutrophil migration and immune function ( |
|
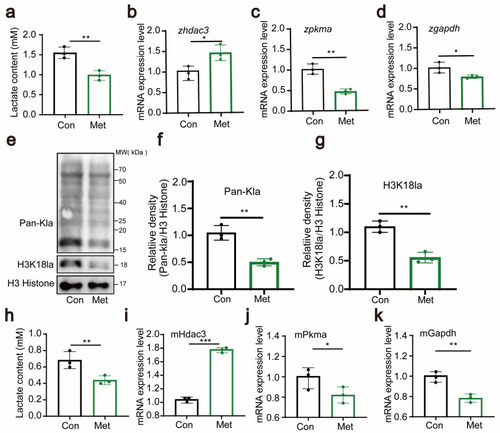
Metformin downregulates H3K18 lactylation. ( |
|
H3K18 lactylation affects neutrophil recruitment by increasing the levels of reactive oxygen species ( |