- Title
-
Differential inflammation responses determine the variable phenotypes of epilepsy induced by GABRG2 mutations
- Authors
- Sui, J., Zhan, L., Ji, S., Wu, W., Chen, Y., Yun, F., Liang, W., Wang, J., Cao, M., Shen, D., Zhang, Q.
- Source
- Full text @ CNS Neurosci Ther
|
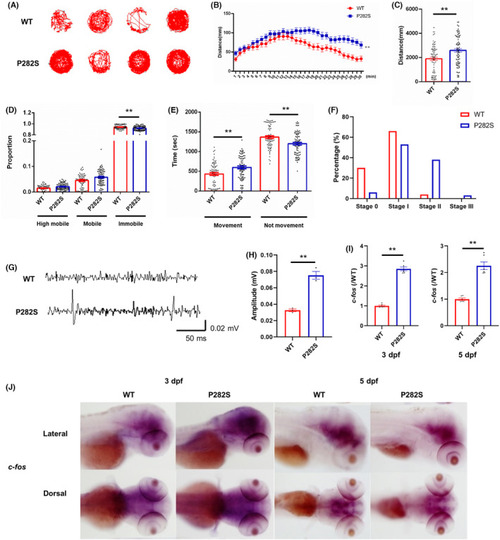
The establishment of Tg( |
|
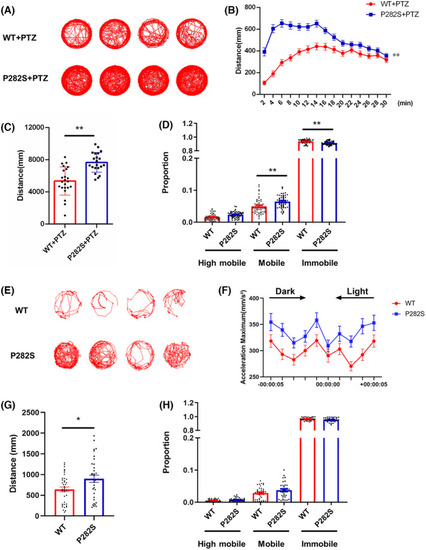
The locomotor activity changes of Tg( |
|
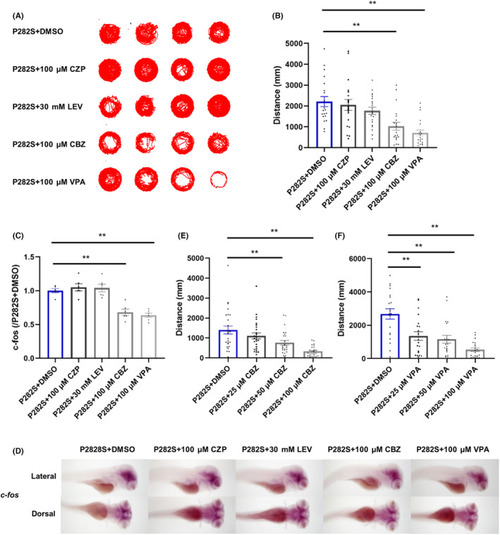
The effects of different traditional AEDs in Tg( |
|
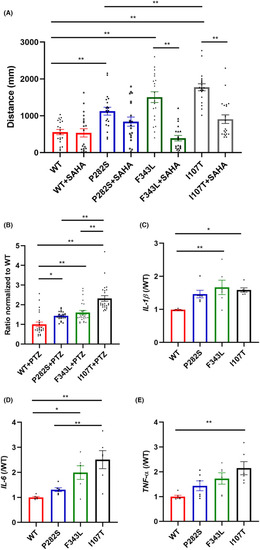
The comparison of behavioral activity, production of pro‐inflammatory factors, and response to SAHA treatment in different transgenic zebrafish. (A) The total distance of the P282S, F343L, and I107T transgenic zebrafish larvae traveled with 2.5 μM SAHA treatment. ** |
|
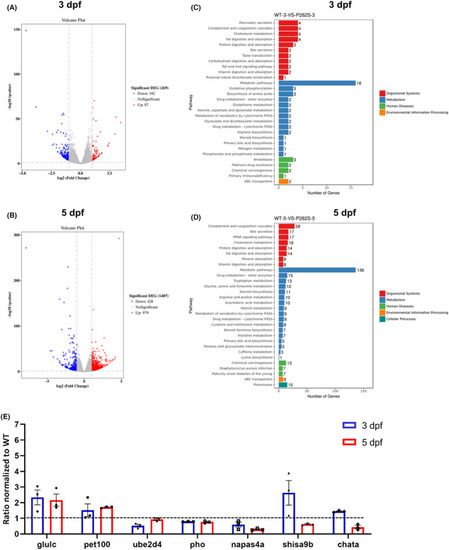
Brain transcriptional profile of Tg( |
|
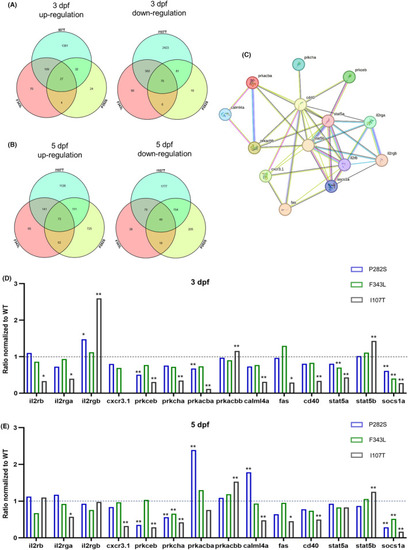
Differentially expressed genes in different mutant |