- Title
-
MEndoB, a chimeric lysin featuring a novel domain architecture and superior activity for the treatment of staphylococcal infections
- Authors
- Roehrig, C., Huemer, M., Lorgé, D., Arn, F., Heinrich, N., Selvakumar, L., Gasser, L., Hauswirth, P., Chang, C.-.C., Schweizer, T.A., Eichenseher, F., Lehmann, S., Zinkernagel, A.S., Schmelcher, M.
- Source
- Full text @ MBio
|
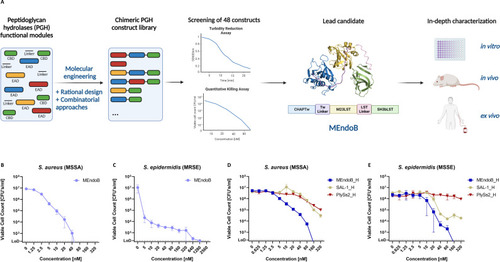
Selection of MEndoB from a library of chimeric staphylococcal PGHs. A chimeric PGH library of 48 constructs was cloned, expressed, and characterized ( |
|
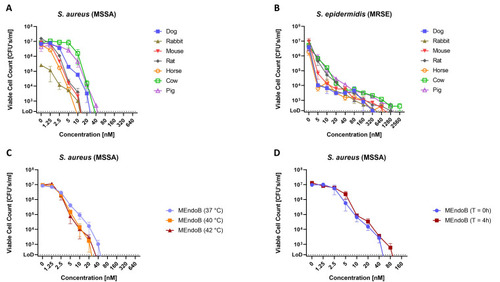
MEndoB kills different staphylococcal species in various animal sera, at elevated temperatures, and remains stable upon storage for 4 h at 37°C in human serum. MEndoB activity in qKAs at 37°C against |
|
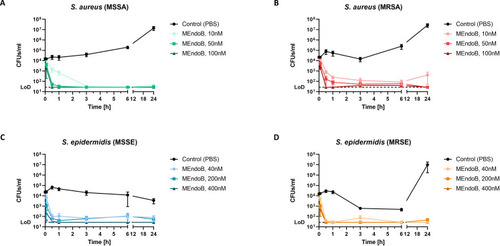
MEndoB effectively kills staphylococcal species in human whole blood. Time kill curves of different |
|
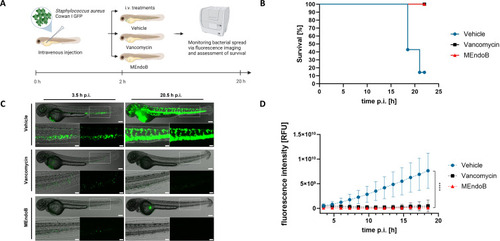
MEndoB rescues zebrafish larvae infected with PHENOTYPE:
|
|
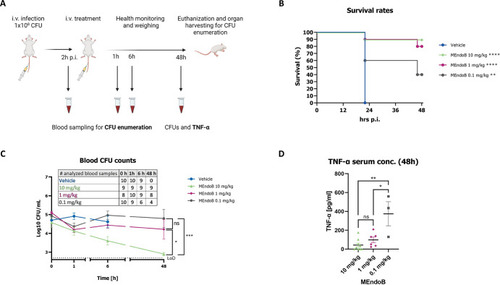
Treatment with MEndoB results in dose-dependent survival of up to 90% in a lethal mouse bacteremia model. Schematic representation of the experimental procedure (A). Survival rates of mice infected with |