Fig 1
- ID
- ZDB-FIG-240215-118
- Publication
- Roehrig et al., 2024 - MEndoB, a chimeric lysin featuring a novel domain architecture and superior activity for the treatment of staphylococcal infections
- Other Figures
- All Figure Page
- Back to All Figure Page
|
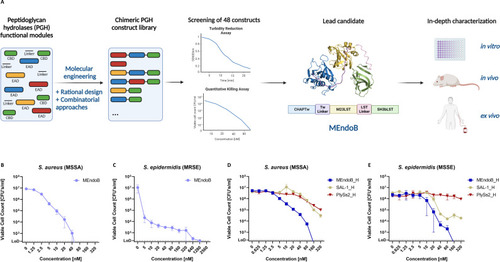
Selection of MEndoB from a library of chimeric staphylococcal PGHs. A chimeric PGH library of 48 constructs was cloned, expressed, and characterized ( |