- Title
-
Colonization of larval zebrafish (Danio rerio) with adherent-invasive Escherichia coli prevents recovery of the intestinal mucosa from drug-induced enterocolitis
- Authors
- Flores, E., Dutta, S., Bosserman, R., van Hoof, A., Krachler, A.-.M.
- Source
- Full text @ mSphere
|
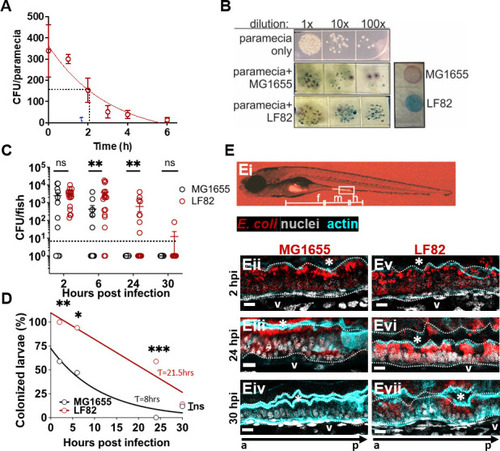
AIEC LF82 colonizes the larval zebrafish intestine better than MG1655. ( |
|
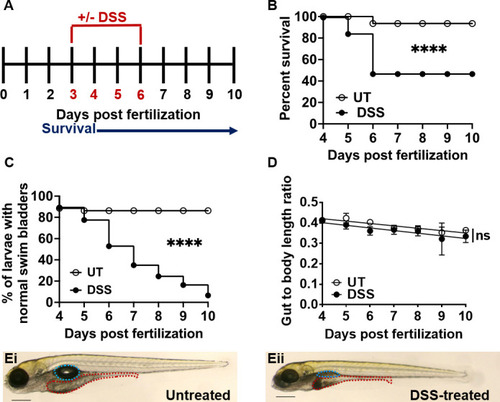
Larval zebrafish treated with 0.5% DSS have decreased survival and intestinal growth rates. ( |
|
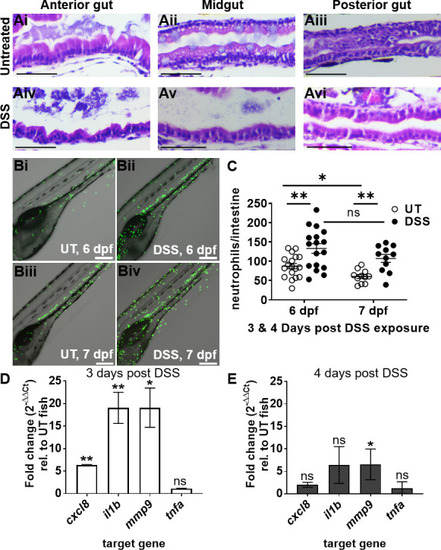
DSS causes intestinal epithelial damage and inflammation consistent with enterocolitis. ( |
|
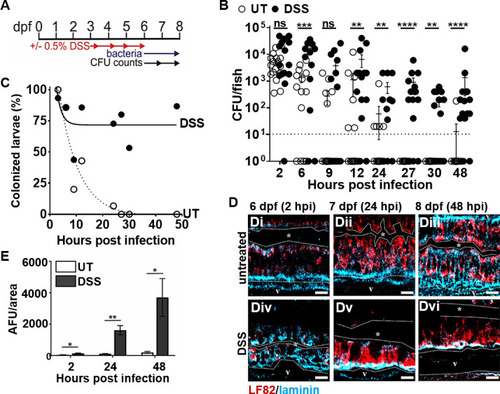
Pre-existing intestinal inflammation enhances the colonization and invasion of AIEC LF82. ( |
|
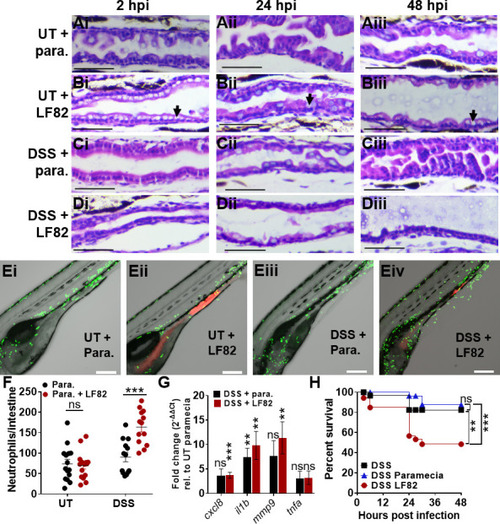
AIEC LF82 exacerbates intestinal inflammation in DSS-treated larvae. ( |
|
Effects of |
|
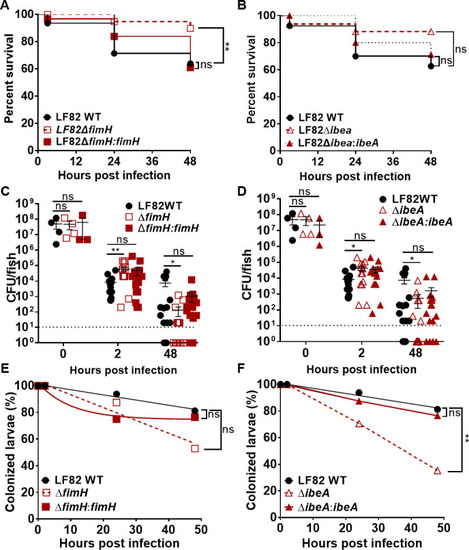
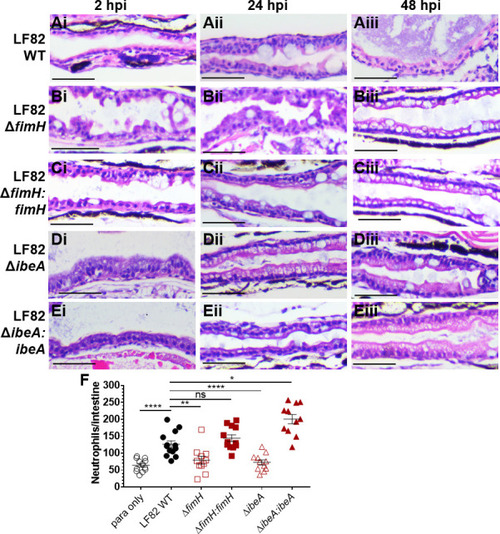
Deletion of |
|
Deletion of |