- Title
-
Photochemically controlled activation of STING by CAIX-targeting photocaged agonists to suppress tumor cell growth
- Authors
- Ding, C., Du, M., Xiong, Z., Wang, X., Li, H., He, E., Li, H., Dang, Y., Lu, Q., Li, S., Xiao, R., Xu, Z., Jing, L., Deng, L., Wang, X., Geng, M., Xie, Z., Zhang, A.
- Source
- Full text @ Chem Sci
|
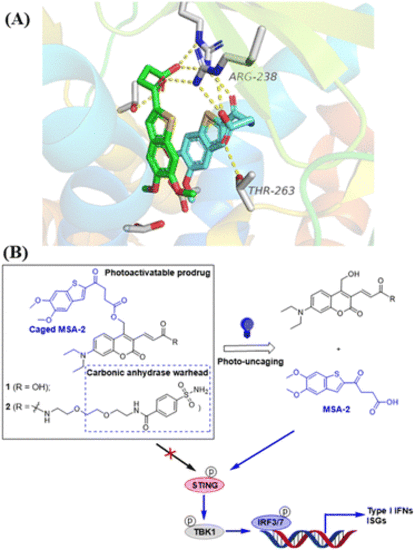
Design of photo-activatable STING agonists. (A) Co-crystal structure analysis of MSA-2 bound to human STING protein (PDB: 6UKM26) identifies the terminal oxobutanoic acid as the critical structural motif for the activation of STING, evidenced by several key hydrogen bond interactions (orange dashed line). (B) STING agonist MSA-2 is caged with DEACM PPGs placed at the terminal oxobutanoic acid, which prevents the activation of the STING signalling. Irradiation with blue light at 450 nm removes the caging group to release MSA-2, which activates STING to induce the phosphorylation of the downstream signalling. |
|
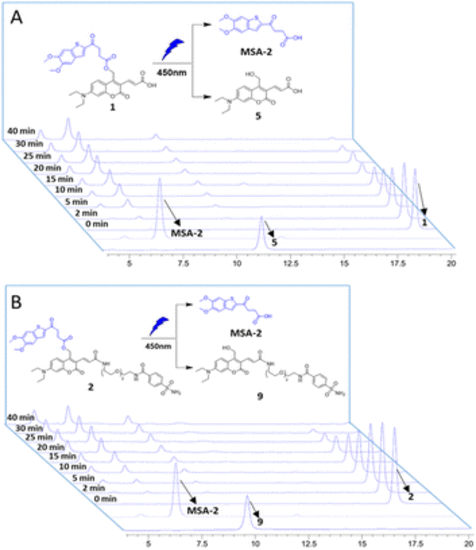
Photouncaging of compounds 1 and 2 (0.5 mM) in CH3CN/PBS buffer. HPLC analysis of compounds 1 (A) and 2 (B) uncaging with 450 nm blue light (intensity: 6.7 nm W cm−2), exhibiting efficient photochemical conversion to the desired MSA-2 in a time-dependent manner. Samples with light irradiation for different times are stacked. The peaks of compounds MSA-2, 1, 2, 5 and 9 are labelled with arrows. |
|
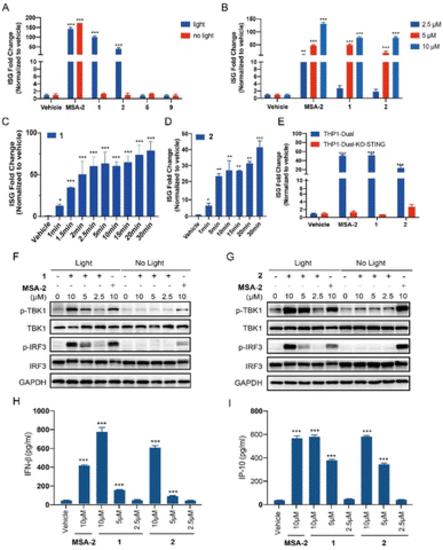
Photo-controllable activation on the STING downstream signalling pathway under blue light. (A) THP1-Dual cells were treated with each compound (MSA-2, 1, 2, 5, and 9) at 10 μM or vehicle (0.1% DMSO), in the presence or absence of blue light (450 nm) for 15 min and then incubated for 24 h. (B) Dose-dependent activation on the ISG reporter of compounds 1 and 2 in THP1-Dual cells under blue light irradiation for 15 min followed by incubation for 24 h. (C) Time-dependent activation on the ISG reporter of compound 1 (5 μM) in THP1-Dual cells under blue light (40 mA, 3.35 mW cm−2) for indicated times, followed by incubation for 24 h. (D) Time-dependent activation on the ISG reporter of compound 2 (5 μM) in THP1-Dual cells under blue light (80 mA, 6.7 mW cm−2) for indicated times, followed by incubation for 24 h. (E) Activation of compounds 1 and 2 (5 μM) on the ISG reporter was dependent on STING under light irradiation for 15 min. The ISG fold change was calculated relative to the vehicle control, and the tests were carried out in triplicate. (F and G) THP1-Dual cells were treated with the indicated concentration of compounds (MSA-2, 1 and 2) with or without 15 min blue light exposure, and then incubated for 4 h. The expression of proteins was determined by western blotting. (H) IFN-β secretion of THP1-Dual cells upon treatment with compounds (MSA-2, 1 and 2) for 24 h after 15 min of blue light exposure. (I) IP-10 secretion of THP1-Dual cells upon treatment with compounds (MSA-2, 1 and 2) for 24 h after 15 min of blue light exposure. Results are expressed as mean ± SEM from two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, as compared to the vehicle using one-way ANOVA. |
|
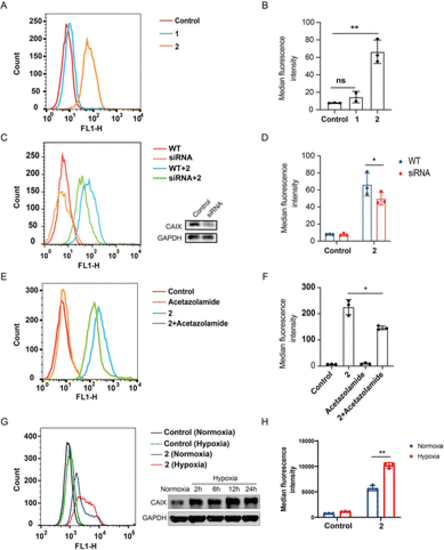
Compound 2 binds to CAIX of CT26 colon cancer cells. (A) Flow cytometric analysis of CT-26 cells treated with 1 and 2 (10 μM), respectively; (B) median fluorescence intensity of (A); (C) flow cytometric analysis of wild type and CAIX knock-down CT-26 cells treated with 2 (10 μM) and western blotting analysis of the CAIX knock-down cells by siRNA; (D) median fluorescence intensity of (C); (E) flow cytometric analysis of CT26 cells treated with acetazolamide (10 μM) and 2 (10 μM), respectively, as well as the acetazolamide-preincubated CT26 cells treated with 2; (F) median fluorescence intensity of (E). (G) CT26 cells were cultured under normoxia and hypoxia (0.5% O2), and 24 h later the cells were incubated with or without 2 (10 μM) and subjected to flow cytometry analysis, besides the expression of CAIX was determined by western blotting at different time points; (H) median fluorescence intensity of (G). Results are expressed as mean ± SEM from two independent experiments (*p < 0.05, **p < 0.01, t-test). |
|
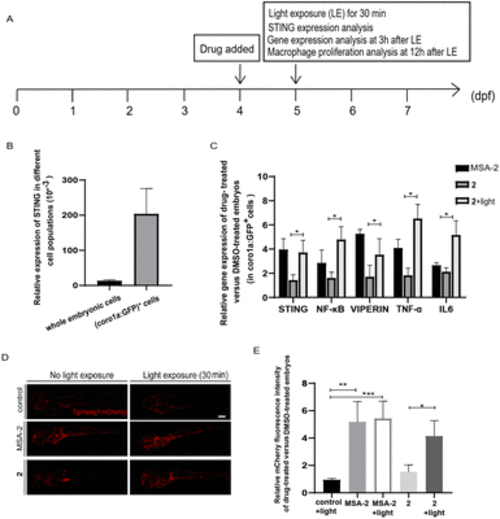
Photo-triggered activation of the STING signalling pathway by 2 in zebrafish. (A) The time scheme for the experiments in (B)–(E). MSA-2 (40 μM) and 2 (10 μM) were added to the embryo medium accordingly. (B) STING is highly expressed in Tg (coro1a:EGFP)+ cells (labelling macrophages and neutrophils). (C) Relative expression of the genes involved in the STING signalling activation in embryos after MSA-2 and 2 treatment with or without light irradiation compared to the DMSO-treated embryos in sorted coro1a:EGFP-labelled cells. Results are expressed as mean ± SEM from three independent experiments, n = 50 embryos per condition (*p < 0.05, t-test). (D) Representative confocal images of Tg (mpeg1:mCherry) embryos (mCherry fluorescence labelling macrophages) after DMSO, MSA-2 and 2 treatment with or without light irradiation. (E) Summary of relative mCherry fluorescent intensity from (D). Results are expressed as mean ± SEM from three independent experiments, n = 15 embryos per condition (***p < 0.001, **p < 0.01, *p < 0.05, t-test). Scale bar in (D): 125 μm; dpf, days post fertilization. |
|
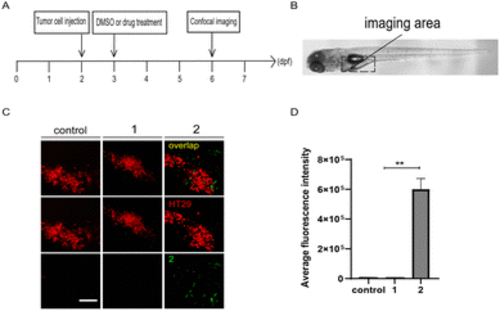
Compound 2 targets tumor cells in zebrafish xenografts. (A) The time scheme for the experiments in (C) and (D). 1 (10 μM) and 2 (10 μM) were added to the embryo medium accordingly. (B) Scheme of the tumor cell injection site and subsequent imaging area. (C) Representative confocal images of HT29 tumor cells, compounds 1 and 2 in zebrafish xenografts at 6 dpf. (D) Summary of the fluorescent intensity of compounds 1 and 2 from (C), obtained by the excitation wavelength of 450 nm (λex = 450 nm). Results are expressed as mean ± SEM from three independent experiments, n = 6–10 embryos per condition (**p < 0.01, t-test). Scale bars in (C): 25 μm; dpf, days post fertilization. |
|
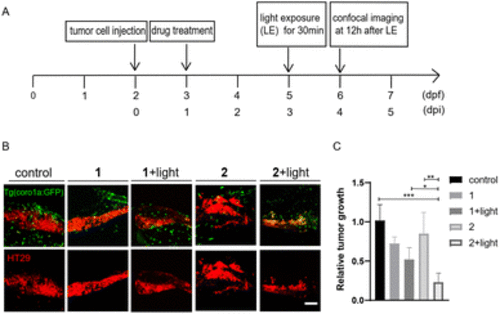
Photo-triggered suppression of tumor cells by 2 in zebrafish xenografts. (A) The time scheme for the experiments in (C)–(F). MSA-2 (40 μM) and 2 (10 μM) were added to the embryo medium accordingly. (B) Scheme of the tumor cell injection site and subsequent imaging area. (C) Representative confocal images of CT26 tumor cells and innate immune cells from Tg (coro1a:GFP) zebrafish xenografts at 4 dpi treated with MSA-2, 2 and 2 irradiated by light. (D) Relative tumor growth at 4 dpi versus 1 dpi. Results are shown as the mean ± SEM from multiple experiments, n = 9 embryos per group (***p < 0.001, ****p < 0.0001, t-test). (E) Representative confocal images of HT29 tumor cells and innate immune cells from Tg (coro1a:GFP) zebrafish xenografts at 4 dpi treated with MSA-2, 2 and 2 irradiated by light. (F) Relative tumor growth at 4 dpi versus 1 dpi. Results are shown as the mean ± SEM from multiple experiments, n = 9 embryos per group (***p < 0.001, ****p < 0.0001, t-test). Scale bars in (C) and (E): 50 μm; dpf, days post fertilization; dpi, days post injection |
|
Compound 2 displayed better antitumor effects than 1 in zebrafish xenografts. (A) The time scheme for the experiments in (B) and (C). 1 (10 μM) and 2 (10 μM) were added to the embryo medium accordingly. (B) Representative confocal images of HT29 tumor cells and innate immune cells from Tg (coro1a:GFP) zebrafish xenografts at 4 dpi treated with MSA-2, 1, 2 or 1 and 2 irradiated by light. (C) Relative tumor growth at 4 dpi versus 1 dpi. Results are shown as the mean ± SEM from multiple experiments, n = 6–9 embryos per group (***p < 0.001, **p < 0.01, *p < 0.05, t-test). Scale bar: 25 μm; dpf, days post fertilization; dpi, days post injection. |