- Title
-
Grhl3 promotes retention of epidermal cells under endocytic stress to maintain epidermal architecture in zebrafish
- Authors
- Phatak, M., Kulkarni, S., Miles, L.B., Anjum, N., Dworkin, S., Sonawane, M.
- Source
- Full text @ PLoS Genet.
|
DIC images of individual wild-type sibling (A, C, E, G, I) and |
|
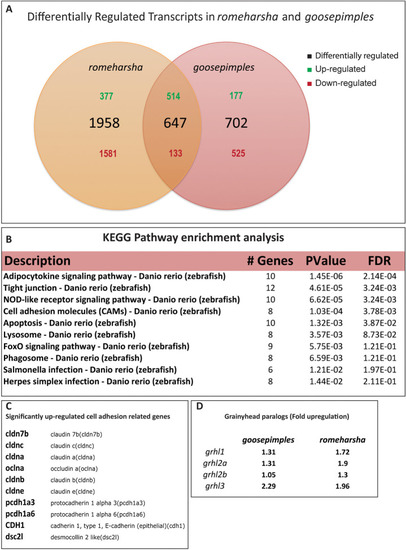
Venn diagram showing the numbers of differentially regulated transcripts in |
|
Bright field images of control morphant (A) and |
|
Bright field images of embryos at 28 hpf injected with control MO (A), myoVb MO (B), grhl3 MO (C), and grhl3 MO; myoVb MO (D). Black arrowheads indicate cell debris accumulated inside the chorion of grhl3 and grhl3;myoVb double morphant embryos, presumably due to epidermal cell shedding. Representative images of the head region of zebrafish larvae injected with control MO (E), myoVb MO (F), grhl3 MO (G), and grhl3;myoVb double morphant (H) at 36 hpf. Zoomed in images of the dorsal head region of myoVb morphant (I) and grhl3;myoVb double morphant (J) embryos, respectively. Representative images of wild-type siblings injected with control MO (K) and myoVb MO (L) and grhl3(-31/-31) mutants injected with control MO (M) and myoVb MO (N). Zoomed in images of dorsal head region of wild-type siblings (O) and grhl3 (-31/-31) mutants (P) injected with myoVb MO. Note the presence of rounded cells in myoVb MO in F, I, L, O—indicated by red arrowheads—and their absence in myoVb; grhl3 double deficient embryos in H, J, N, P. Representative confocal images of the peridermal cells from the stated genetic conditions at 48hpf (Q-T). Note the presence of rounded cells in myoVb MO indicated by red triangles. Box and whisker plot (U) showing quantitation of cell circularity in control, grhl3 MO, myoVb MO, and grhl3;myoVb MO at 48hpf. Each column represents cell circularities of 25 cells each from four embryos per condition (total 100). Grey dotted line represents the cell circularity threshold used for classifying round cells. Red numbers indicate % cells having cell circularity values above threshold. myoVb MO embryos show significantly higher median cell circularity than that in control and grhl3;myoVb MO (Kruskal Wallis One way ANOVA p<0.05). For all comparisons and statistical significance please refer to S2 Data. |
|
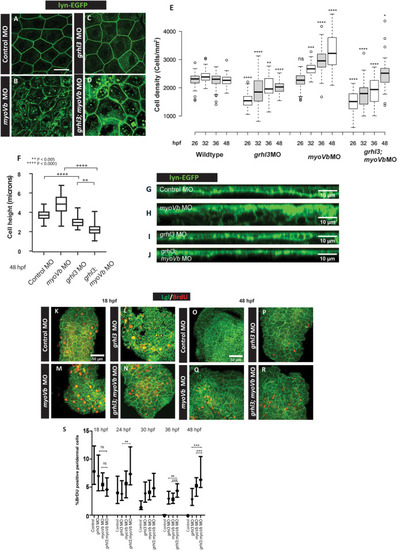
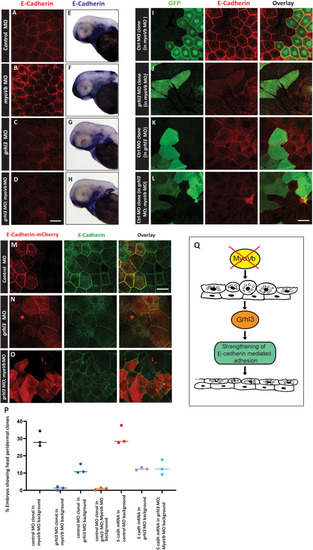
A box and whisker plot (A) showing quantification of cell shedding by control morphant, grhl3 morphant, myoVb morphant, and grhl3;myoVb double morphant embryos during time windows: 12–18 hpf, 18–24 hpf, 24–30 hpf, and 30–36 hpf. Note that post 24 hpf, cell shedding in grhl3 morphants is comparable to that in myoVb morphants, whereas that in grhl3;myovb double morphants remains significantly higher (data presented as box plots showing median and IQR, n = 20, N = 5, one-way ANOVA, p<0.0001). Quantification of peridermal rosette structures in the dorsal head region. Box and whisker plot shows rosette densities (rosettes/mm2) at various developmental stages (B). Numbers in red indicate % embryos showing rosettes. Median rosette density is comparable at 26 hpf in all conditions. However, at 32 hpf, 36 hpf and 48 hpf, grhl3;myoVb morphants show significantly higher rosette density and occurrence than those in either grhl3 or myoVb morphants. Statistical significance was calculated using Kruskal Wallis one-way ANOVA with Dunn’s post hoc test. Representative confocal images (B’) showing rosettes indicated by yellow arrowheads in stated genetic conditions. Confocal images of dorsal head periderm showing clones injected with control MO, myoVb MO, grhl3 MO, and grhl3+myoVb MO and marked by GFP (green) and stained for E-cadherin (red) at 48 hpf (C, D, E, F). Scale bar = 50 μm. The percentage of embryos showing peridermal clones was calculated as readout of cell retention probability in the periderm. The dot plot shows quantification of clone retention at 12 hpf and 48 hpf (G); individual points represent % embryos showing peridermal clones in a set, horizontal line denotes median. n ~ 60, N = 3. Note that grhl3 and grhl3;myoVb double morphant clones show lower retention compared to those of control morphant or myoVb morphant clones at 48 hpf. Square brackets (in A and B) indicate comparisons and asterisks represent statistically significant difference. Only relevant comparisons in A and B are shown. For all comparisons and statistical significance please refer to S2 Data. PHENOTYPE:
|
|
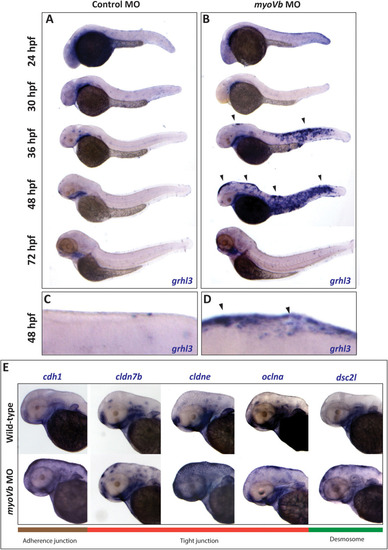
Representative confocal images of cldnB:lynEGFP embryos (A-D), injected with control MO, EXPRESSION / LABELING:
PHENOTYPE:
|
|
Representative confocal images of dorsal head periderm of control (A), |