- Title
-
Sirtuin 3 is essential for host defense against Mycobacterium abscessus infection through regulation of mitochondrial homeostasis
- Authors
- Kim, Y.J., Lee, S.H., Jeon, S.M., Silwal, P., Seo, J.Y., Hanh, B.T.B., Park, J.W., Whang, J., Lee, M.J., Heo, J.Y., Kim, S.H., Kim, J.M., Song, G.Y., Jang, J., Jo, E.K.
- Source
- Full text @ Virulence
|
SIRT3 is essential for host defense against mycobacterial infection |
|
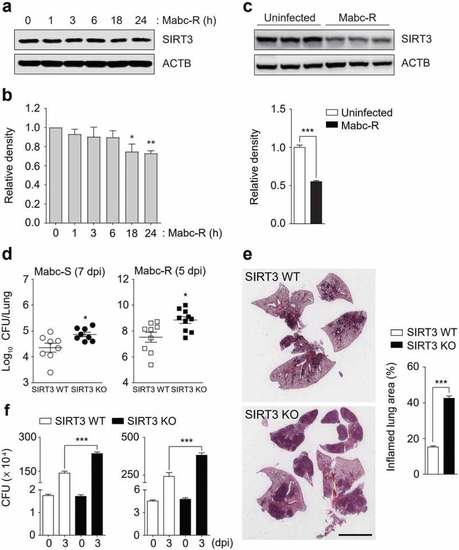
SIRT3 is required to control pathological inflammation and mitochondrial damage during Mabc-R infection. (a and b) SIRT3 WT and KO mice ( |
|
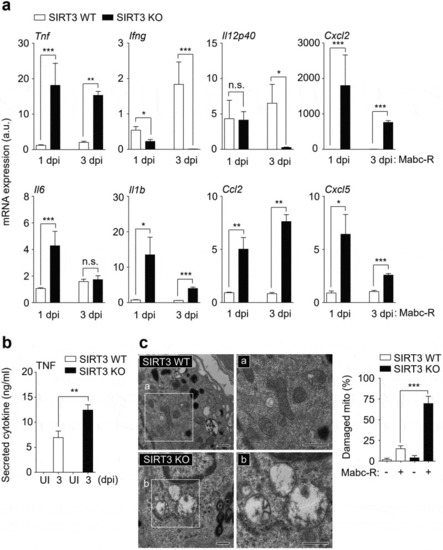
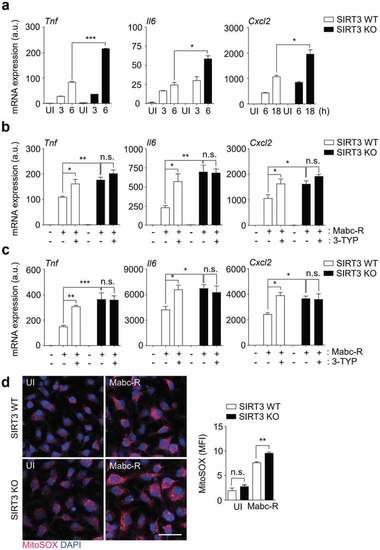
SIRT3 is essential for the amelioration of proinflammatory cytokine expression and controlling mitochondrial ROS production in BMDMs during Mabc-R infection. (a) BMDMs from SIRT3 WT and KO mice were infected with Mabc-R (MOI = 3) and incubated for 3, 6, or 18 h. (b and c) BMDMs (b) and PMs (c) were prepared from SIRT3 WT and KO mice, and were infected with Mabc-R (MOI = 3) in the presence or absence of 3-TYP (50 µM) for 3 h and quantitative real-time PCR analysis for |
|
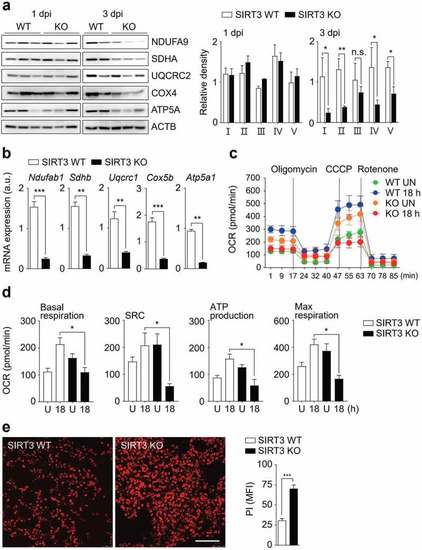
SIRT3 is required for mitochondrial OXPHOS function and attenuation of cell death during Mabc-R infection. (a and b) SIRT3 WT and KO mice ( |
|
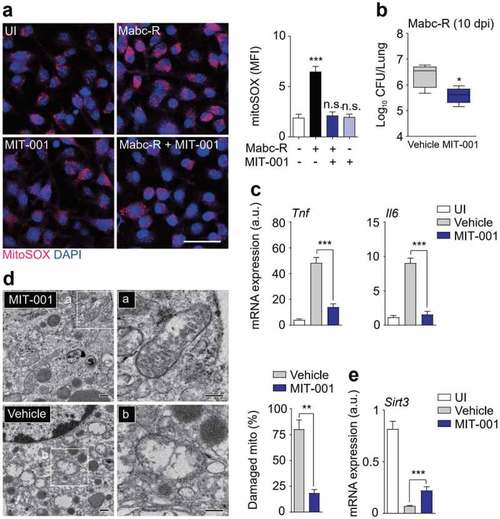
Administration of MIT-001 in mice led to a protective effect against Mabc-R infection. (a) WT BMDMs were infected with Mabc-R (MOI = 5) for 2 h in the presence or absence of MIT-001 (20 µM) and subjected to MitoSOX Red staining (Left, representative images; right, quantitative analysis). Scale bar, 50 μm. (b-e) WT mice ( |
|
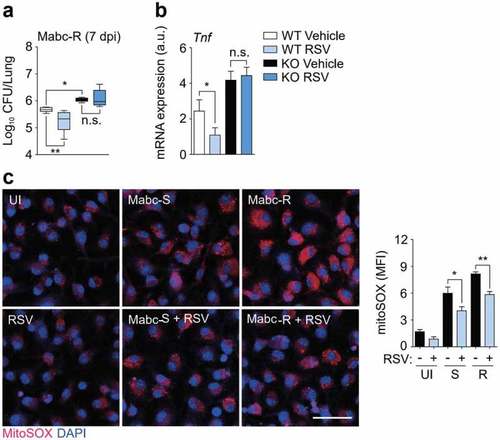
SIRT3 activation by RSV enhances antimicrobial responses and ameliorates mitochondrial oxidative stress during Mabc-R infection. (a and b) SIRT3 WT and KO mice ( |
|
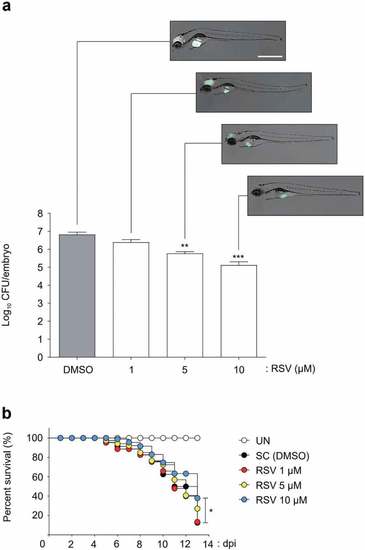
Efficacy of the RSV in Mabc-R infected ZF. (a) The panels show representative dissemination of Mabc-R-mWasabi in ZF. The fluorescent bacterial dissemination and bacterial burden after treatment with different doses of RSV (1, 5, or 10 ÁM) were assessed. Statistical significance was determined by ANOVA using Tukey?s multiple comparison test. **P < 0.01, ***P < 0.001. Scale bar, 0.5 mm. (b) Survival curve of embryos infected with Mabc-R. The embryos were infected with Mabc-R, followed by treatment with RSV or solvent control (S. C.; DMSO, 1%). As a positive control, ZF without infection was used. RSV, Resveratrol; UNT, untreated. *P < 0.05. PHENOTYPE:
|