- Title
-
The Olfactory Organ Is Populated by Neutrophils and Macrophages During Early Development
- Authors
- Palominos, M.F., Whitlock, K.E.
- Source
- Full text @ Front Cell Dev Biol
|
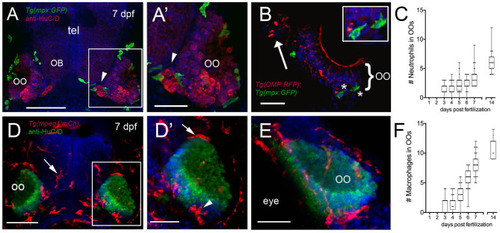
Neutrophils and macrophages are found in developing sensory systems. |
|
Neutrophils and macrophages populate the olfactory system of juvenile animals. |
|
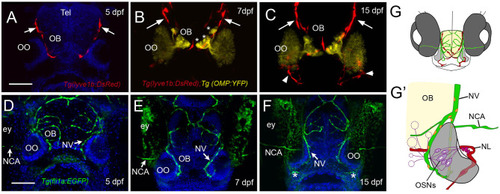
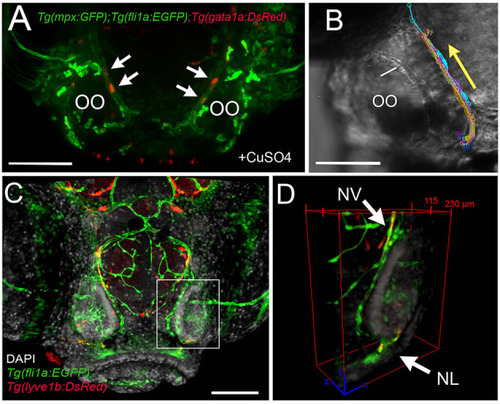
The developing olfactory organs have an extensive blood-lymphatic system. Lyve1b:DsRed+ lymphatic vessels (red) at 5 dpf (A), 7 dpf (B), and 15 dpf (C). (A–C) Lyve1b:DsRed+ lymphatic vasculature (red, arrows) extends from the dorsal brain toward the olfactory organs (OO). (B,C) Lymphatic vessels extend to region of olfactory sensory neurons (OMP:YFP+), yellow, asterisks) in olfactory bulb (OB). (C) At 15 hpf, nasal lymphatic vasculature (red, arrowheads) is now visible wrapping around the posterior OO and associating with the ventral lateral OOs (G). Fli1a:EGFP+ blood vasculature (green) at 5 dpf (D), 7 dpf (E), and 15 dpf (F). Blood vessels (green) forming nasal vein (NV) and nasal ciliary artery (NCA) are present at 5 dpf. The NV extends ventrally (E) encircling the OO (F). (G) Diagram of head of 15 dpf larva showing telencephalon (green) and olfactory organs (gray). (G′) Olfactory organ (gray) summarizing blood (green) and lymphatic (red) vasculature. Nuclei of NV (orange) are positive for both lyve1b:DsRed and fli1a:EGFP. (A,D,E,F): DAPI (blue). OO: olfactory organ, OB: olfactory bulb, ey: eyes. Scale bars: (A–C) = 200 μm, (D–F) = 200 μm. |
|
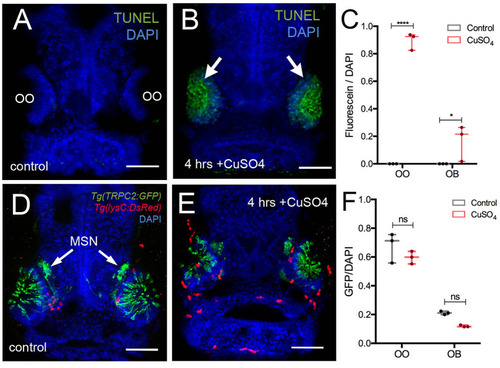
Copper exposure induces cell death and subsequent regeneration in the olfactory sensory system. |
|
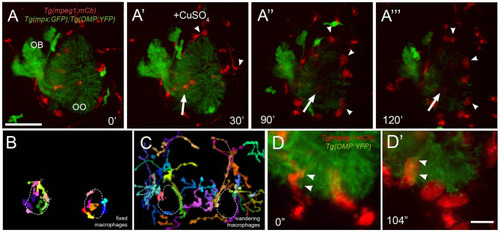
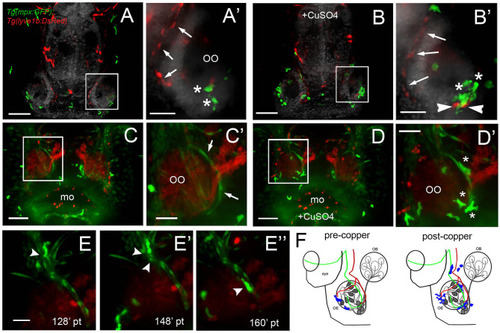
Exposure to copper induces migration of neutrophils. |
|
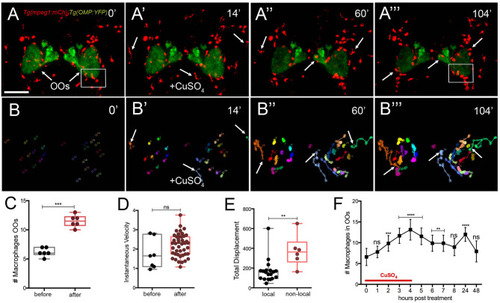
Exposure to copper induces migration response of macrophages in the olfactory organ. |
|
Exposure to copper reveals distinct classes of macrophages. |
|
During copper exposure neutrophils migrate in association with blood vasculature to reach to the developing olfactory organ. |
|
The nasal vein as the primary route to the olfactory organ during development. |
|
Summary of neutrophil and macrophage responses to copper-induced damage in the larval olfactory organ (OO). The blood vasculature (BV, green) wraps the olfactory organ (gray) and the lymphatic vasculature (LV, red) extends along the ventral posterior aspect. In untreated animals (before copper), there are local neutrophils (blue) and macrophages (pink) associated with the OO. In response to damage (after copper exposure) of the olfactory sensory neurons (OSNs, dark gray), non-local neutrophils and macrophages migrated to the OO. Neutrophils migrated in association with the BV, and both neutrophils and macrophages were seen associated with the LV. Macrophages changed to a more rounded morphology as they engulfed debris of dying OSNs. |