- Title
-
The Axenfeld-Rieger Syndrome Gene FOXC1 Contributes to Left-Right Patterning
- Authors
- Chrystal, P.W., French, C.R., Jean, F., Havrylov, S., van Baarle, S., Peturson, A.M., Xu, P., Crump, J.G., Pilgrim, D.B., Lehmann, O.J., Waskiewicz, A.J.
- Source
- Full text @ Genes (Basel)
|
Allelic consequence of foxc1-targeted CRISPR/Cas9 mutagenesis: (A) a schematic representation of foxc1aua1017 and foxc1bua1018 mutations, with 7 and 40 base pair deletions (yellow highlighting) upstream of the DNA-binding Forkhead domain; (B) the predicted sequences of the first 40 amino acids translated from wildtype (WT) and mutant proteins with sequence identity (black highlighting), where the foxc1aua1017 allele produces a truncated 39-residue protein with loss of sequence homology from amino acid 10 and the foxc1bua1018 allele produces a truncated 28-residue protein with loss of sequence homology from amino acid 19; and (C) PCR genotyping from the gDNA template resolving the respective deletions in foxc1a and foxc1b. (FHD, Forkhead domain; UTR, untranslated region; CDS, coding sequence.). |
|
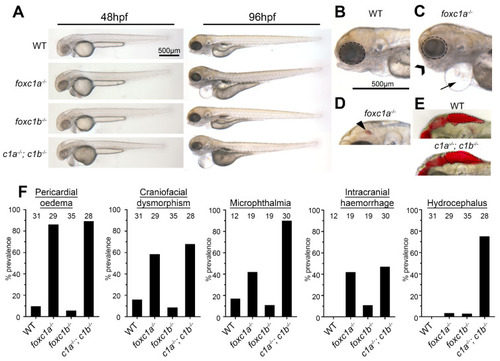
Foxc1 mutants exhibit multiple developmental defects. (A) foxc1 single mutants are largely indistinguishable from WT (wildtype) controls at 48 hpf (left panels), whereas foxc1a−/−; foxc1b−/− double homozygotes display hydrocephalus and oedema. By 96 hpf, no changes are observed in foxc1b−/− homozygotes, however; foxc1a−/− homozygotes and foxc1a−/−; foxc1b−/− double homozygotes display pericardial oedema (compare B and C, as highlighted by the arrow), microphthalmia (dotted circle), and craniofacial dysmorphism (chevron). At this stage, a subset of mutants also displayed intracranial hemorrhage (arrowhead in panel D), with foxc1a−/− homozygotes having greater frequency than foxc1b−/− homozygotes (42% vs. 11%, respectively), and (E) foxc1a−/−; foxc1b−/− double homozygotes present with hydrocephalus. (F) Quantification reveals that these defects are incompletely penetrant and generally more prevalent in double than single homozygotes (the number of embryos analyzed is shown above each bar). In the case of hydrocephalus, only the double homozygotes display an appreciable frequency of this phenotype. |
|
Decreased dosages of foxc1 result in multi-organ situs defects. (A) In situ hybridization with myl7 at 48 hpf revealed aberrant cardiac situs (O-loop (green); L-loop (red)) compared with normal D-loop (blue). The prevalence of aberrant situs is increased in foxc1a, foxc1b, and double homozygotes (* p = 0.016, * 0.036, *** < 0.001 respectively, Fisher’s exact test) when compared to WT siblings. (B) At the same stage, the normal arrangement (solitus, blue) of the gut is left-sided liver (l) and right-sided pancreas (p); however, the incidence of abnormal (ambiguous, green) gut situs was significantly greater in double homozygotes (** p = 0.003, Fisher’s exact test). |
|
Foxc1a mRNA overexpression causes cardiac situs defects in a dose-dependent manner: (A–C) myl7 in situ hybridization of 75 pg foxc1a mRNA-injected embryos at 48 hpf, with representative images of the three cardiac looping morphologies provided (v, ventricle; a, atrium); (D) quantification of cardiac situs in control and foxc1a mRNA-injected embryos revealing an increasing prevalence of anomalous cardiac looping with increasing amounts of foxc1a mRNA; and (E) quantification of embryo situs defects in embryos injected with 75 pg of foxc1a mRNA. Statistical significance was apparent in comparisons between foxc1a/b and mCherry controls (cardiac: p = 0.0002 and 0.0012; gut: p < 0.0001 and 0.0205. mCherry vs. foxc1a and foxc1b respectively. One-way ANOVA and Dunnett’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001). |
|
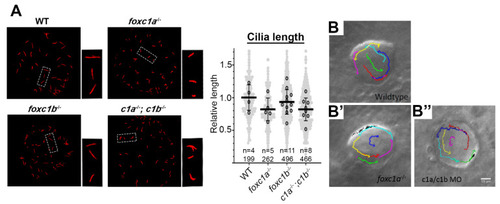
Loss of foxc1 does not significantly change cilia length or left–right organizer flow. (A) Acetylated α-tubulin immunostaining of left–right organizer (LRO) cilia revealed that average cilia length was not significantly altered in foxc1 mutants (foxc1a−/−, 82%; foxc1b−/−, 93%; foxc1a−/−; foxc1b−/−, 82% of WT length; p = 0.286, ANOVA; 4–11 embryos imaged per condition, all cilia per condition shown in light grey). Tracking of fluorescent bead flow in the LRO revealed unchanged counterclockwise flow between WT (B), foxc1a−/− homozygotes (B’), and foxc1a/foxc1b morphants (B’’). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Foxc1 mutants display loss of lefty2 expression independent of changes to other left–right patterning genes. (A) In situ hybridization with southpaw (spaw) revealed no difference in the prevalence of normal left-sided expression (normal = blue, absent = black, bilateral = green, and right = red) in foxc1 mutants compared to controls (p > 0.1, Fisher’s exact test). (B) Conversely, lefty2 expression was significantly altered in foxc1 mutants. Normal left-sided lefty2 expression was absent more frequently in foxc1a−/− and double foxc1a−/−; foxc1b−/− homozygotes (p = 0.012 and 0.028, respectively) and trended to be absent in foxc1b−/− homozygotes without reaching statistical significance (p = 0.061, Fisher’s exact test) (normal = blue and absent = black). (C) lefty2 expression was significantly abnormal, with the overexpression of foxc1a or foxc1b (p = 0.0007 and 0.0030, respectively, ANOVA and Dunnett’s Test). (D) Analysis of the additional left–right patterning genes lefty1, pitx2c, and elvol6 revealed no differences between WT, and foxc1a−/−; foxc1b−/− double homozygotes. (* p < 0.05, ** p < 0.01, *** p < 0.001). EXPRESSION / LABELING:
PHENOTYPE:
|