- Title
-
Effect of AMPK-p53-klf2a Pathway in Hyperglycemia-induced Cardiac Remodeling in Adult Zebrafish
- Authors
- Wang, Q., Luo, C., Lu, G., Chen, Z.
- Source
- Full text @ J Diabetes Investig
|
Streptozocin (STZ) induces hyperglycemia in zebrafish. (a) Blood glucose assay analysis of blood glucose in vivo continues for 30 days. The fish were injected with STZ in a dose of 350 mg/kg and maintained in different days. Values >200 mg/dL were considered hyperglycemic. (b) Quantitative polymerase chain reaction analysis of glucose transporter (GLUT)1, GLUT2 and GLUT12 in hyperglycemic zebrafish hearts. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was measured as the control. (c) Western blot analysis of GLUT1, GLUT2 and GLUT12 in hyperglycemic zebrafish injected with STZ. GAPDH was used as the control. (d) Survival analysis of the hyperglycemic fish and control fish in 30 days. (e) Body mass analysis of the hyperglycemia zebrafish model. Each experiment was repeated a minimum of three times. **Highly significant difference (P < 0.01) in a two‐tailed Student’s t‐test. |
|
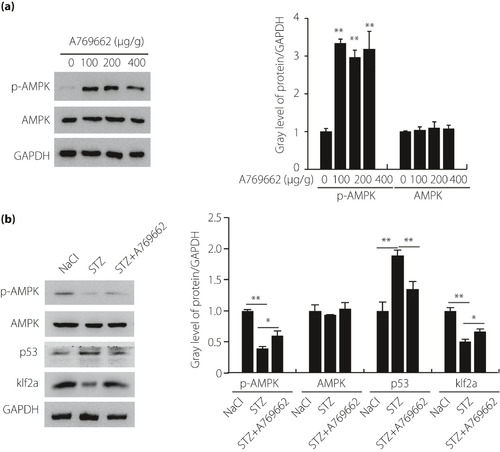
The adenosine monophosphate‐activated protein kinase (AMPK) pathway was involved in the hyperglycemic zebrafish. (a) Real‐time polymerase chain reaction and western blot analysis of the expression of AMPK in different concentrations of A769662 (n = 21 fish per group). (b) Real‐time polymerase chain reaction and western blot analysis of the expression of AMPK, p53 and Krüppel‐like factor 2a (klf2a) in hyperglycemic zebrafish and A769662 intervention zebrafish compared with the control group (n = 21 fish per group). Each experiment was repeated a minimum of three times. *Statistically significant difference (P < 0.05), **Highly significant difference (P < 0.01) in a two‐tailed Student’s t‐test. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; p‐AMPK, phospho‐adenosine monophosphate‐activated protein kinase; STX, streptozocin. |
|
Hyperglycemia induced muscular disarray, myofibril loss, vacuolization, mitochondrial condensation and apoptosis activation. (a) Transmission electron microscopy image verified muscular disarray, myofibril loss, vacuolization and mitochondrial condensation in hyperglycemic zebrafish compared with the control group (n = 21 field, repeated five times); scale bars, 2 μm. (b) Hematoxylin–eosin staining analysis of the heart in hyperglycemic zebrafish and A769662 intervention zebrafish compared with the control group after 21 days; scale bars, 100 μm. Quantification analysis of ventricular myocardial density in hematoxylin–eosin‐stained hearts between the three groups. (c) Terminal deoxynucleotidyl‐transferase‐mediated dUTP nick‐end‐labeling (TUNEL) assay analysis of cell apoptosis in the heart of hyperglycemia zebrafish and A769662 intervention zebrafish compared with the control group zebrafish; scale bars, 50 μm. Each experiment was repeated a minimum of three times. **Highly significant difference (P < 0.01) in a two‐tailed Student’s t‐test. STX, streptozocin. |
|
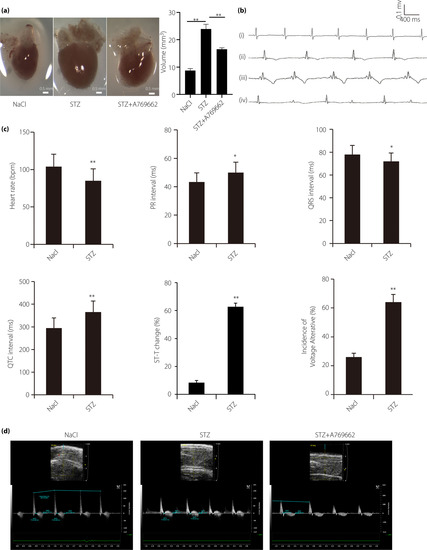
Cardiac dysfunction was involved in hyperglycemic zebrafish. (a) Streptozocin (STZ) treatment induces enlarged heart in zebrafish, which is partially canceled by A769662, adenosine monophosphate‐activated protein kinase activator; scale bar, 0.5 mm. (b) Typical electrocardiogram diagram; (i) normal electrocardiogram; (ii) escape rhythm representative diagram; (iii) T‐wave inversion; and (iv) “voltage alternation” diagram. (c) Electrocardiogram parameters were significantly different in the two groups, such as heart rate, PR interval, QRS interval, QTc interval, ST‐T change and voltage‐alterative. (d) Typical B‐mode echocardiography images of hyperglycemic zebrafish, control zebrafish and A769662 intervention zebrafish to evaluate ventricular morphology and function. Image data were automatically generated using the Vevo 2100 Workstation Software package (VisualSonics, Toronto, Canada). (n = 21 fish). *P < 0.05, **P < 0.01 compared with the wild‐type group. |
|
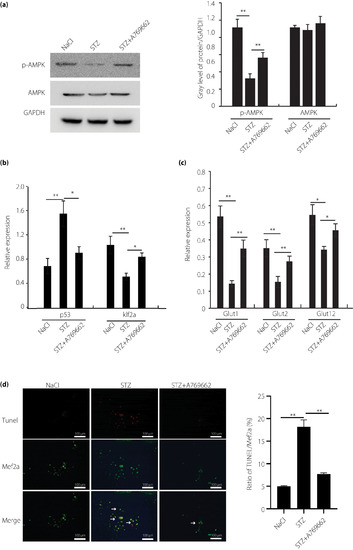
Adenosine monophosphate‐activated protein kinase (AMPK)–p53–Krüppel‐like factor 2a (klf2a) pathway involved in hyperglycemic zebrafish cardiomyocytes. (a) Real‐time polymerase chain reaction and western blot analysis of the expression of AMPK in hyperglycemic and control zebrafish cardiomyocytes, and control with A769662 intervention in vitro. (b) Real‐time polymerase chain reaction analysis of the expression of p53 and klf2a in hyperglycemic and control zebrafish cardiomyocytes, and control with A769662 intervention in vitro. (c) Real‐time polymerase chain reaction analysis of the expression of glucose transporters (Glut) in hyperglycemic and control zebrafish cardiomyocytes, and control with A769662 intervention in vitro. (d) Terminal deoxynucleotidyl‐transferase‐mediated dUTP nick‐end‐labeling (TUNEL) assay analysis of cell apoptosis in hyperglycemic and control zebrafish cardiomyocytes, and control with A769662 intervention cardiomyocytes cultured in vitro; scale bars, 100 μm. Each experiment was repeated a minimum of three times. *Statistically significant difference (P < 0.05), **Highly significant difference (P < 0.01) in a two‐tailed Student’s t‐test. p‐AMPK, phospho‐adenosine monophosphate‐activated protein kinase; STX, streptozocin. |
|
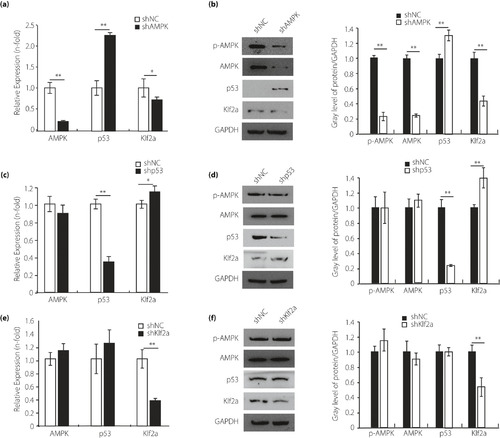
The upstream and downstream relationship among the adenosine monophosphate‐activated protein kinase (AMPK) pathway in the zebrafish cardiomyocytes. (a,b) Quantitative polymerase chain reaction and western blot analysis of AMPK pathway in the cardiomyocytes transfected with short hairpin AMPK (shAMPK). Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was measured as the control. (c,d) Quantitative polymerase chain reaction and western blot analysis of the AMPK pathway in the cardiomyocytes transfected with short hairpin p53 (shp53). GAPDH was measured as the control. (e,f) Quantitative polymerase chain reaction and western blot analysis of the AMPK pathway in the cardiomyocytes transfected with short hairpin klf2a (shklf2a). GAPDH was measured as the control. Each experiment was repeated a minimum of three times. *Statistically significant difference (P < 0.05), **Highly significant difference (P < 0.01) in a two‐tailed Student’s t‐test. p‐AMPK, phospho‐adenosine monophosphate‐activated protein kinase; STX, streptozocin. |