- Title
-
Characterization of Hspb8 in Zebrafish
- Authors
- Dubińska-Magiera, M., Niedbalska-Tarnowska, J., Migocka-Patrzałek, M., Posyniak, E., Daczewska, M.
- Source
- Full text @ Cells
|
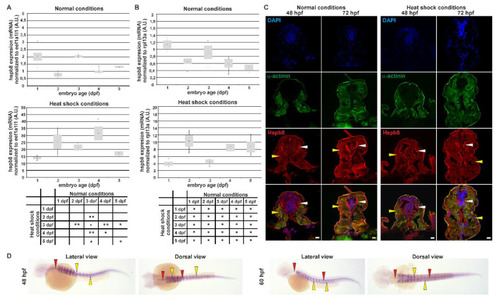
Tissue-specific expression and localization of Hspb8 during zebrafish development under normal and heat shock conditions. A and B. Real-time quantitative PCR (RT qPCR) of |
|
Tissue-specific and subcellular localization of Hspb8 during zebrafish development. ( |
|
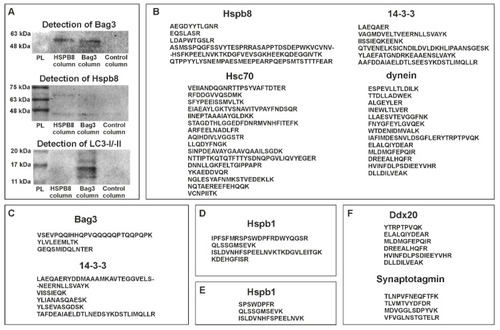
Protein partners of zebrafish Hspb8. ( |
|
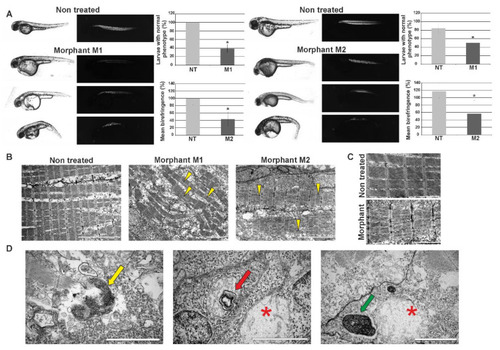
Effects of morpholino-mediated knockdown of zebrafish |
|
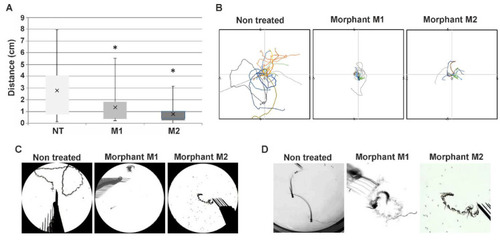
Altered swimming behavior in zebrafish embryos with decreased |