- Title
-
Allergic Reactions and Immunity in Response to Tick Salivary Biogenic Substances and Red Meat Consumption in the Zebrafish Model
- Authors
- Contreras, M., Pacheco, I., Alberdi, P., Díaz-Sánchez, S., Artigas-Jerónimo, S., Mateos-Hernández, L., Villar, M., Cabezas-Cruz, A., de la Fuente, J.
- Source
- Full text @ Front Cell Infect Microbiol
|
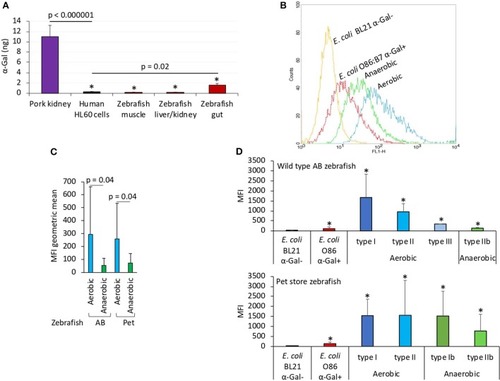
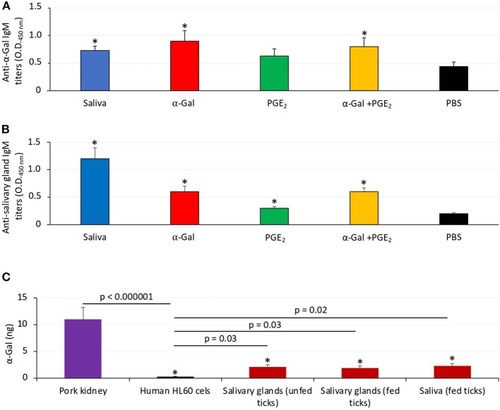
The α-Gal content is similar in humans and zebrafish. The α-Gal content was determined in zebrafish tissues and gut bacterial microbiota and in |
|
Zebrafish develop antibodies against tick α-Gal and proteins. |
|
Experimental design. Experiments were designed and performed to evaluate the allergic reactions and immune response in zebrafish treated with tick saliva and salivary components and in response to red meat consumption. |
|
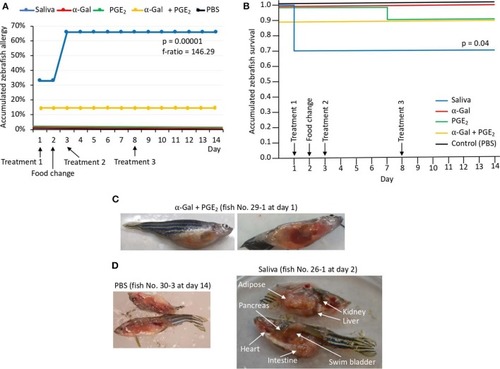
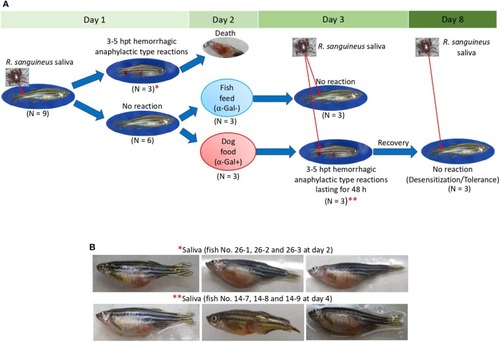
Zebrafish injected with tick saliva develop hemorrhagic anaphylactic-type reactions (Experiment 1). |
|
Zebrafish injected with tick saliva and fed with red meat develop hemorrhagic anaphylactic-type reactions and desensitization (Experiment 1). |
|
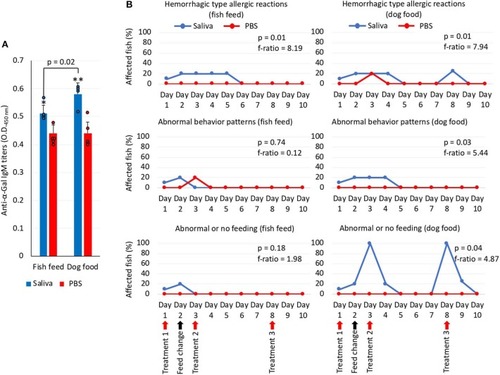
α-Gal levels in dog food and fish feed and correlation analysis between anti–α-Gal IgM antibody levels and allergic reactions to tick saliva. |
|
Zebrafish injected with tick saliva and fed with red meat develop allergic reactions and abnormal behavior and feeding patterns (Experiment 2). |
|
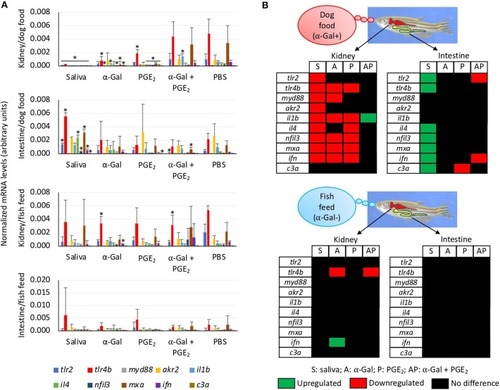
Tissue-specific differences in the immune response of zebrafish injected with tick saliva and fed with red meat (Experiment 1). |
|
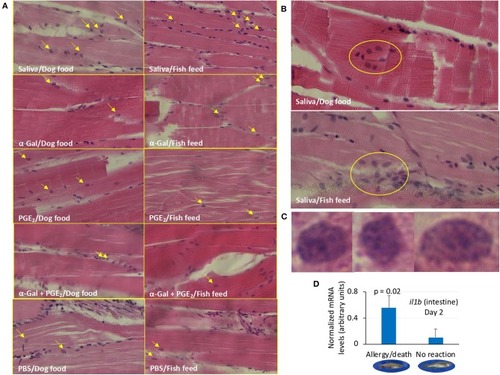
Granulocyte profile in zebrafish (Experiment 1). |
|
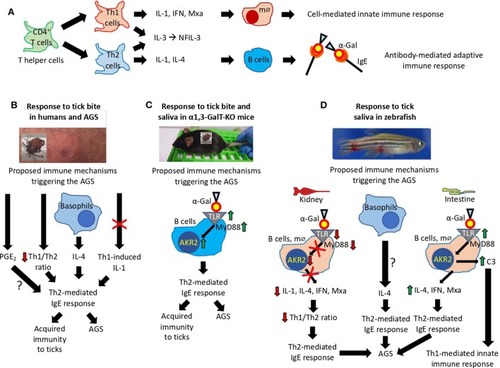
Proposed mechanisms triggering the AGS. |