- Title
-
25-hydroxycholesterol impairs neuronal and muscular development in zebrafish
- Authors
- Jamadagni, P., Patten, S.A.
- Source
- Full text @ Neurotoxicology
|
25 Hydroxycholesterol affects development and is lethal upon chronic exposure at higher doses: The percentage of embryos surviving decreases (A) and hatching among the surviving embryos is delayed (B) with increase in the concentration of 25−HC. (C) morphological defects in development of the embryos at 2 dpf (days post fertilization), viewed and imaged using a bright field microscope. Defects get severe with increasing concentration of 25-HC. (D) The percentage of dead embryos and larvae with abnormal phenotypes (with heart edema and curved tails) increases in a dose dependent manner upon chronic exposure. |
|
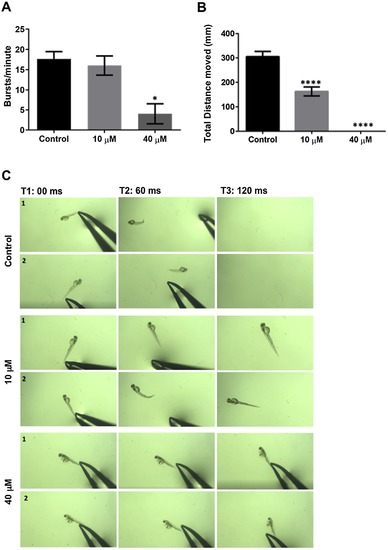
25-HC affects early motility in zebrafish larvae: (A) Representation of the number of early spontaneous coiling of the larvae at 20 hpf (hours post fertilization) treated with 25-HC. There is a decrease in a dose dependent manner. (B) The distance moved by 5 dpf larvae upon treatment with 25-HC were assessed using the Ethiovision software, show a highly significant reduction in a dose dependent manner. (C) The figure represents motion snapshots of touch response in two representative trials (1,2) (N = 10) over 120 miliseconds (ms). At 40 μM, the larvae also have very little to no escape response to touch at 2 dpf. All comparisons are performed with control, each trial has a N of 7 for control and treated over 4 trials. * represents a significance of p < 0.05, **** represents a significance of p < 0.01. Significance was determined using one-way ANOVA and all data are represented as Mean ± SEM. |
|
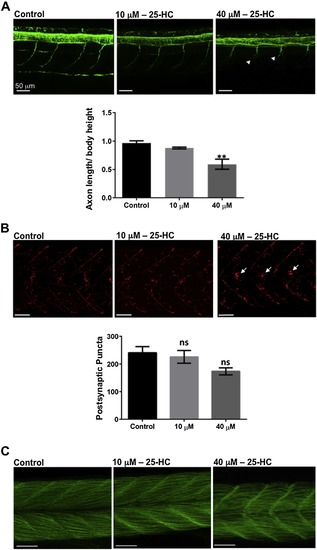
Effects of 25-HC on the neuromuscular system in zebrafish larvae: (A) The spinal cord of control and treated larvae at 2 dpf, stained for acetylated α-tubulin, showed a significant reduction in the axon length of the motor neurons (white arrow heads) at 40 μM. (B) Staining for α-bungarotoxin in the muscles of treated and control larvae show no significant difference in the number of postsynaptic puncta. (C) Phalloidin staining of muscles in 25-HC-treated fish and controls at 2 dpf. All comparisons are performed with control, each trial has a n of 7 for control and treated over 4 trials. ** represents a significance of p < 0.01, ns: not significant. Significance was determined using one-way ANOVA and all data are represented as Mean ± SEM. |
|
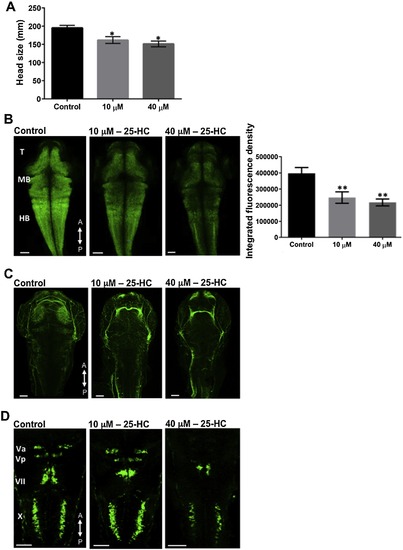
25-HC administration alters brain development in zebrafish: (A) Head size comparison between the treated and un-treated larvae at 2 dpf. Characterizing the larval brains at 2 dpf using Tg(huc:GFP) (B) and Tg(Isl1:GFP) (D) lines showed a decrease in the neuronal population and a defective development of the cranial nerves, in a dose dependent manner. (C) Larvae stained for acetylated α-tubulin at 2 dpf, showed reduced axon network clusters in the brain. All comparisons are performed with control, each trial has a n of 7 for control and treated over 4 trials, * represents a significance of p < 0.05, ** represents a significance of p < 0.01. Significance was determined using one-way ANOVA and all data are represented as Mean ± SEM. T: Telencephalon, MB: Midbrain, HB, Hindbrain. A, P represent anterior and posterior side of the head respectively. |
|
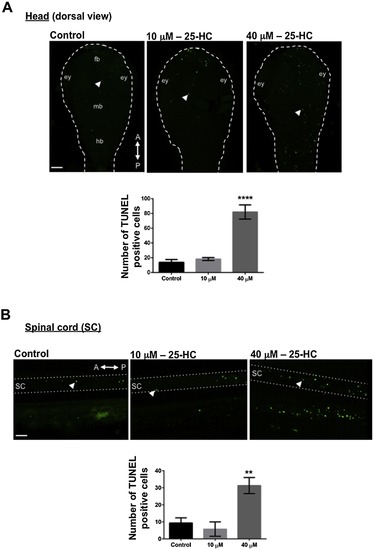
25-HC administration causes increased cell death: TUNEL staining at 2 dpf in treated and untreated larvae showed an increased cell death in the head (white arrow heads) (A) and the spinal cord (SC; white arrow heads) (B) upon 25-HC treatment in a dose dependent manner. Ey: eye; fb:forebrain; mb: midbrain; hb: hindbrain; sc: spinal cord. Each trial has a n of 7 for control and treated, repeated over 3 trials. A,P: Anterior and posterior side of the embryos respectively. |
|
25-HC administration had no effect on Mauthner cells but caused cell death: (A) An immunostaining of 3A10, specific for Mauthner cells, showed no difference in the development of the cells. Acridine orange staining at 2 dpf in treated and un-treated larvae showed an increased cell death in the brain (white arrow heads) (B) and spinal cord (white arrow heads)(C) in a dose dependent manner. Each trial has a n of 7 for control and treated, repeated over 3 trials. A,P: Anterior and posterior side of the embryos respectively. |
|
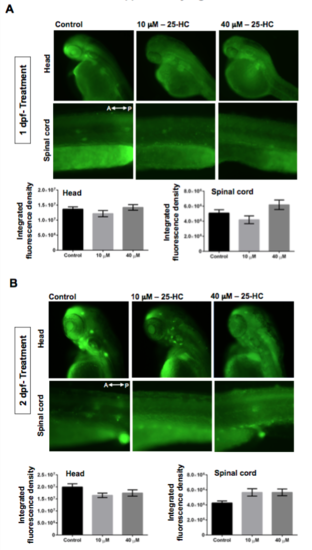
Administration of 25-HC at stages post fertilization does not induce cell death: Acridine Orange staining on larvae treated with 25-HC at 1 dpf (A) and 2 dpf (B) showed no cell death in the brain and spinal cord. The graphs represent quantification of the net fluorescence upon Acridine orange staining. Each trial has a n of 7 for control and treated, repeated over 3 trials. A,P: Anterior and posterior side of the embryos respectively. |
|
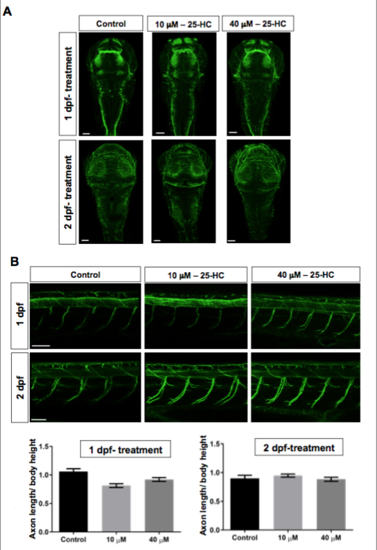
Effects of administration of 25-HC at stages post on neural network in the brain and on primary motor axons: A staining for acetyl Tubulin, specific for neuronal axons, on larvae treated with 25-HC at 1 dpf and 2 dpf. Neural network in the brain are disorganized in 25- HC treated fish while the axon out-growth of motor neurons in the spinal cord between the treated and control larvae are not different. Each trial has a n of 7 for control and treated, repeated over 3 trials. |