- Title
-
Novel roles for the radial spoke head protein 9 in neural and neurosensory cilia
- Authors
- Sedykh, I., TeSlaa, J.J., Tatarsky, R.L., Keller, A.N., Toops, K.A., Lakkaraju, A., Nyholm, M.K., Wolman, M.A., Grinblat, Y.
- Source
- Full text @ Sci. Rep.
|
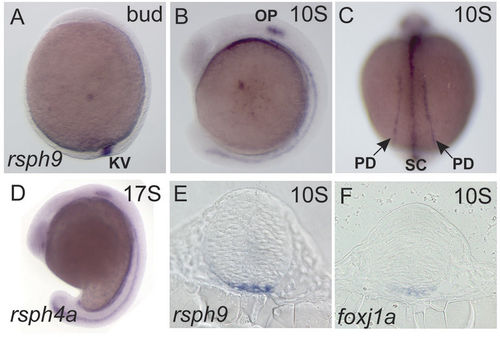
Rsph9 and Rsph4a are expressed in the motile ciliogenic embryonic domains. Wild-type embryos were stained by ISH to identify cells that express rsph9 (A–C,E), rsph4a (D) and foxj1a (F). (A,B,D) are whole mount embryos in lateral view, anterior to the left. (C) Is a dorsal view. (E,F) are transverse sections at the level of midbrain. KV: Kupffer’s vesicle; OP: otic placode; PD: pronephric duct; SC: ventral spinal cord. EXPRESSION / LABELING:
|
|
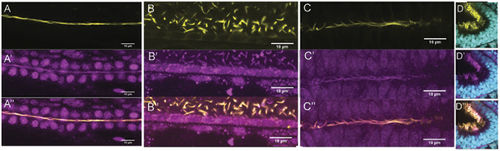
Rsph9 protein is enriched in cilia. Wildtype embryos were stained with an anti-Rsph9 antibody (magenta) and acetylated α-tubulin (yellow). (A) Pronephric duct. (B) Ventral spinal cord. (C) Ventral midbrain. (D) Olfactory pit, nuclei are visualized with DAPI (cyan). All images are shown with anterior to the left. (A,B) Are lateral mounts (dorsal at the top). EXPRESSION / LABELING:
|
|
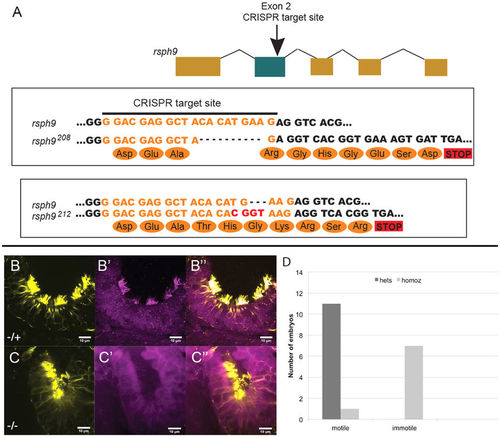
Olfactory cilia require Rsph9 function for motility. (A) Two independent mutant alleles at the rsph9 locus were obtained using CRISPR/Cas9 mutagenesis. (B,C): rsph9208/+ (B) and rsph9208/rsph9208 (C) siblings were fixed at 5 dpf and stained for acetylated α-tubulin (yellow) and Rsph9 (magenta). (D) Chart summarizing olfactory ciliary motility shows reduced motility in homozygous mutants. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
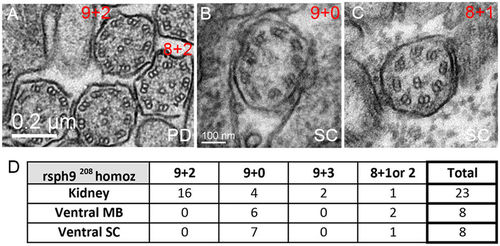
Ultrastructural defects in rsph9 mutant cilia. (A–C): Representative TEM images of cilia from rsph9208/ rsph9208 embryos at 1 dpf. Both the normal 9 + 2 and aberrant 8 + 2 axonemes are present in the pronephric ducts (A). Normal 9 + 0 (B) and aberrant 8 + 1 (C) cilia are found in the ventral spinal cord. (D) Summary of ciliary structures observed by TEM in rsph9208/ rsph9208 embryos surveyed at kidney level (5 embryos, 2 experiments), midbrain level (3 embryos, 1 experiment), and spinal cord level (4 embryos, 2 experiments). PD: pronephric duct; SC: ventral spinal cord. PHENOTYPE:
|
|
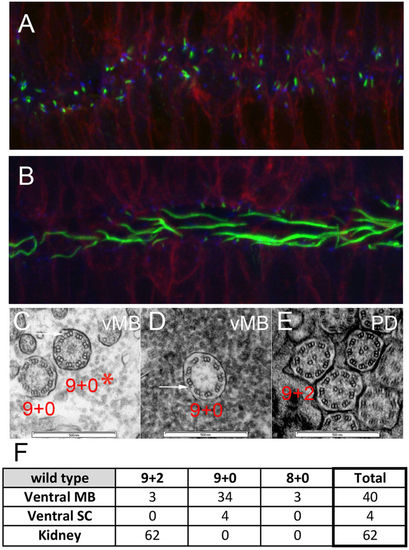
Ventral midbrain and ventral spinal cord produce long cilia with 9 + 0 axonemes. (A,B) Tg(β-actin:mGFP) embryos at 24 hpf were stained with antibodies against acetylated α-tubulin (green) and γ-tubulin (blue) to label ciliary axonemes and basal bodies, respectively. (A) Short cilia at the dorsal midbrain midline. (B) Long cilia at the ventral midbrain midline. (C–E) Wildtype embryos at 1 dpf were processed for transmission electron microscopy and analyzed in transverse sections at the levels of ventral midbrain (C,D) and pronephric duct (E). Arrows point to outer dynein arms. (F) Summary of ciliary structures in the ventral midbrain, spinal canal, and pronephric ducts of wiltype embryos. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
Single antibody controls for rsph9/ ace-tubulin immunostaining (controls for bleed-through) Wild type embryos were stained with an acetylated α-tubulin (A) or anti-Rsph9 antibody (B). Embryos were then incubated with DAPI (cyan) to label nuclei. A-A'': pronephric duct in embryo stained for α-tubulin only. Note absence of ciliary staining on the 568 channel when overexposed. B-'': Pronephric duct in embryo stained for Rsph9 only. Note absence of ciliary staining on the 488 channel, when overexposed. |
|
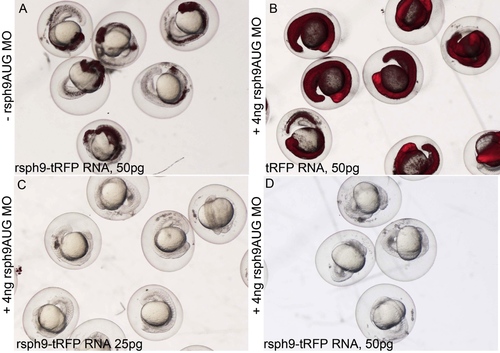
Morpholino designed against the translation start site of Rsph9 blocks translation from exogenous Rsph9 RNA. A-D: Wild-type embryos injected with different combinations of rsph9 MO and rsph9- tRFP mRNA. A: Injection of rsph9-tRFP mRNA alone results in tRFP fluorescence at varying levels between embryos. B: Co-injection of rsph9MO with tRFP mRNA does not prevent tRFP fluorescence. C,D: Co-injection of rsph9MO with rsph9-tRFP mRNA, which contains the binding site of the rsph9MO, prevents expression of rsph9-tRFP, as indicated by absence of RFP fluorescence. |
|
Morpholino knockdown of Rsph9 causes structural axonemal defects. A-H: TEM images of ciliary cross-sections in rsph9 morphant embryos at 1 dpf. Ventral neural cilia with 9+0 axonemes found in the ventral midbrain (A) and spinal cord (B) of uninjected control embryos. Note outer dynein arms (arrow), indicative of ciliary motility. Rsph9-depleted morphants contain aberrant 8+1 cilia in the ventral midbrain (C, D). Pronephric cilia in uninjected controls have 9+2 axonemes (E). Rsph9 morphants contain a mixture of cilia with 9+2 (F), supernumerary central microtubules, e.g. 9+5 (G) and 9+0 (H). I: Summary of TEM analysis of ciliary axonemes in wildtype, control morpholino-injected, and Rsph9 morpholino-injected embryos. PHENOTYPE:
|
|
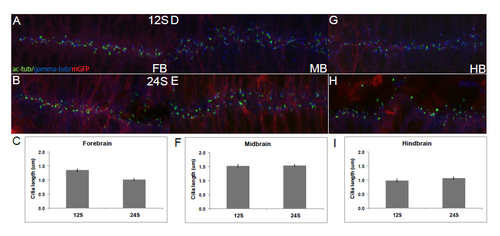
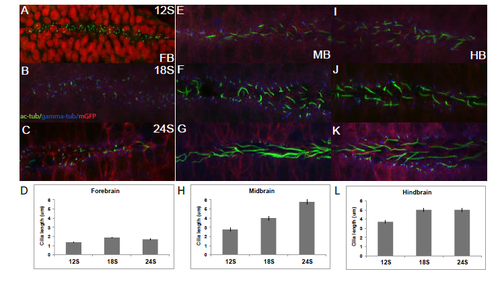
Dorsal cilia are uniform across brain subdivisions and throughout neurulation. Fixed Tg(β-actin:mGFP) embryos stained with antibodies against acetylated α-tubulin (green), gamma-tubulin (blue) and GFP (red). (A,B) Cilia in the dorsal FB do not change in length over time. (C) Average length of dorsal FB cilia at 12S (1.36μm), and 24S (1.02μm). (D,E) Dorsal MB cilia remain unchanged over time. (F) Averaged length at 12S (1.53μm) and 24S (1.54μm). (G,H) Dorsal HB cilia remain unchanged over time. (I) Averaged length at 12S (0.98μm) and 24S (1.07μm). Cilia length was measured in Image J (NIH). Averages were compiled from 20 cilia picked from 10um stacks of the dorsal- and ventral-most regions of the neural tube. A-B, D-E, G-H are stacked confocal images with anterior to the left. Error bars represent standard error of the mean. |
|
Distinct subpopulations of ventral cilia in each brain subdivision increase in length during neurulation. Tg(β-actin:mGFP) embryos stained with antibodies against acetylated α-tubulin (green), gamma-tubulin (blue) and GFP (red), except in A, where nuclei are stained red. (A-C) Cilia in the ventral FB do not change in length over time. (D) Average length of ventral FB cilia at 12S (1.36μm), 18S (1.87μm) and 24S (1.69μm). (E-G) Ventral MB cilia are longer than primary cilia on average and increase in length over time. (H) Average length at 12S (2.76μm), 18S (4μm) and 24S (5.77μm). (I-K) Ventral HB cilia are longer than MB cilia at 12S and increase in length over time. (L) Average length at 12S (3.72μm), 18S (5.02μm) and 24S (5.01μm). A-C, E-G, I-K are stacked confocal images with anterior to the left. Error bars represent standard error of the mean. |
|
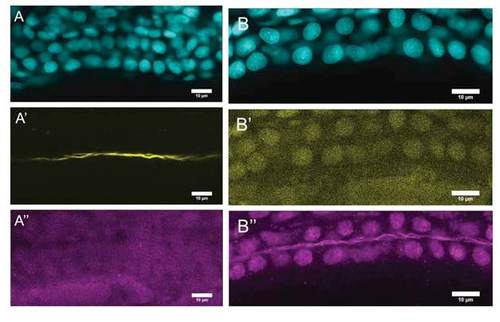
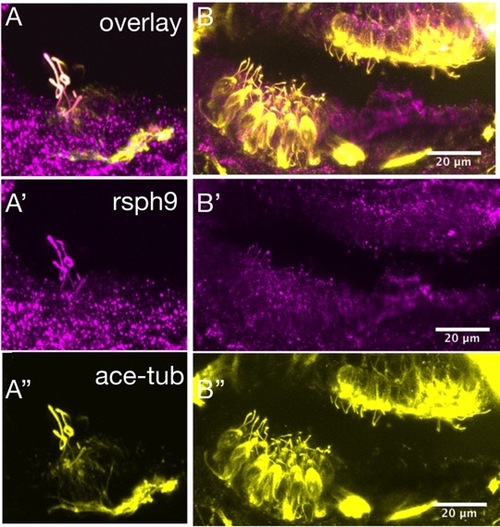
Rsph9 immunoreactivity co-localizes with acetylated tubulin in hair cell kinocilia. Wildtype embryos at 3 dpf were immunostained for acetylated tubulin (yellow) and rsph9 (magenta). A-A'': crista hair cells with kinocilia labeled by acetylated tubulin and rsph9. B-B'': macula hair cells with kinocilia labeled by acetylated tubulin and rsph9. EXPRESSION / LABELING:
|
|
Rsph9 function is not required for kinocilia formation or hair cell mechanotransduction. rsph9208/+ (A,C) and in rsph9208/rsph9208 (B,D) sibling embryos at 3 dpf were immunostained for acetylated tubulin to label kinocilia. A, B: kinocilia of the inner ear lateral crista hair cells. C, D: kinocilia in the lateral line neuromasts. E, F: larvae were derived from a cross between rsph9208/ rsph9208 females and rsph9208/+ males and treated with FM1-43 vital dye at 6 dpf. E (higher magnification in E'): a representative FM1-43 treated rsph9208/+ larva. F (higher magnification in F'): a representative FM1-43 treated rsph9208/rsph9208 larva. PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|