- Title
-
Yap/Taz transcriptional activity in endothelial cells promotes intramembranous ossification via the BMP pathway
- Authors
- Uemura, M., Nagasawa, A., Terai, K.
- Source
- Full text @ Sci. Rep.
|
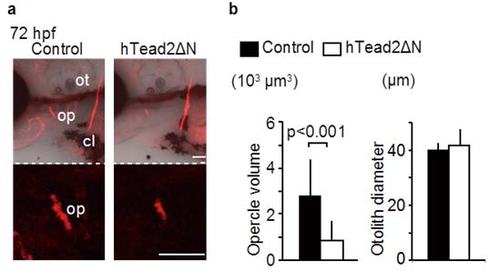
Inhibition of Yap/Taz transcriptional activity in endothelial cells downregulates intramembranous ossification. (a) 293T cells were transfected with pFR-Luc and pcDNA3.1-Gal4-hTead2ΔN for measuring the transcriptional activities of zYap or zTaz. Cells were also introduced with p3xflag-cmv-14-zYap, -zTaz, and pEGFP-hTead2ΔN, as indicated. 24 hours after transfection, cells were harvested and measured the transcriptional activities of zYap or zTaz by luciferase activity. Control cells were introduced pFR-Luc and pcDNA3.1-Gal4-hTead2ΔN with or without pEGFP-hTead2ΔN. Average and SD were calculated from 3 experiments. (b,c) Control Tg(fli1: gal4-vp16) embryos and Tg(fli1: gal4-vp16): (UAS: GFP-htead2ΔN) were stained with alizarin red s, and the volume of the opercle (op), cleithrum (cl), and the diameter of the otolith (ot) at indicated hours post fertilization (hpf) were measured. The Branchiostegal ray (bsr) is also indicated. Average and SD were calculated from more than 13 embryos in each sample. Bar: 50 µm. (d,e) Similar experiments to (b,c) were performed using Tg(fli1: gal4-vp16): (UAS: GFP-hyapN). Average and SD were calculated from more than 11 embryos in each sample. Bar: 50 µm. |
|
Overexpression of Yap/Taz in endothelial cells promotes intramembranous ossification. (a) Control Tg(fli1: gal4-vp16) embryos and Tg(fli1: gal4-vp16): (UAS: GFP-hyap) were stained with alizarin red s, and the volume of the opercle (op) was measured at the indicated hpf. Average and SD were calculated from more than 23 embryos in each sample. Bar: 50 µm. (b) Similar experiments as (a) were performed with Tg(fli1: gal4-vp16): (UAS: GFP-htaz). Average and SD were calculated from more than 35 embryos in each sample. Bar: 50 µm. |
|
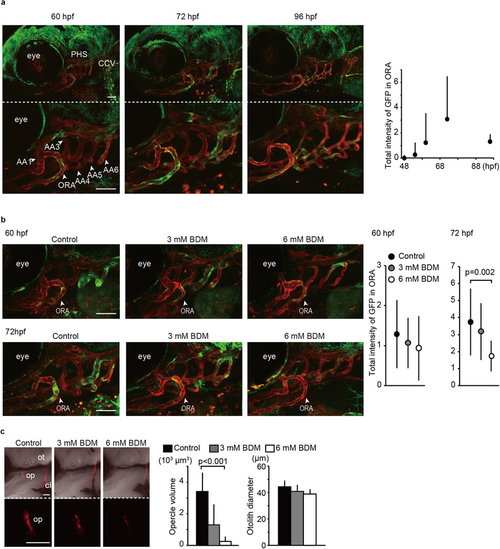
Blood flow is required for Yap/Taz transcriptional activation in ORA and intramembranous ossification. (a) Stacked images of Tg(fli1: gal4-htead2ΔN): (UAS: GFP): (fli1: myr-mCherry) at the indicated hpf are shown. Green color represents Yap/Taz transcriptional activity. Red color represents all endothelial cells. The opercular artery (ORA), primary head sinus (PHS), common cardinal vein (CCV), and branchial arteries (AA) are also indicated. Average and SD of the total GFP intensity in ORA were measured from 20 embryos at each time-point and are shown in the right panel. Bar: 50 µm. (b) Similar experiments to (a) were performed with BDM treatment. Embryos without BDM treatment also shows as control. Tg(fli1: gal4-htead2ΔN): (UAS: GFP): (fli1: myr-mCherry) were treated with BDM from 54 hpf to the indicated hpf. Average and SD of the total GFP intensity in the ORA were measured from more than 18 embryos at each time-point. Bar: 50 µm. (c) Embryos were treated with or without BDM from 54 hpf to 72 hpf, stained with alizarin red s, and the volume of the opercle (op) and the diameter of the otolith (ot) were measured. Embryos without BDM treatment also shows as control. Average and SD were calculated from more than 10 embryos in each sample. Bar: 50 µm. |
|
Yap/Taz transcriptional activation in endothelial cells promotes runx2 expression. (a) Control Tg(fli1: gal4-vp16), Tg(fli1: gal4-vp16): (UAS: GFP-htead2ΔN), and Tg(fli1: gal4-vp16): (UAS: GFP-hyap) were fixed at 60 hpf with paraformaldehyde, and WISH was performed to detect the mRNA of runx2a and osterix. (b) Similar experiments to (a) were performed. Embryos of Tg(fli1: gal4-vp16), were treated with BDM from 54 to 72 hpf at the indicated dosage. |
|
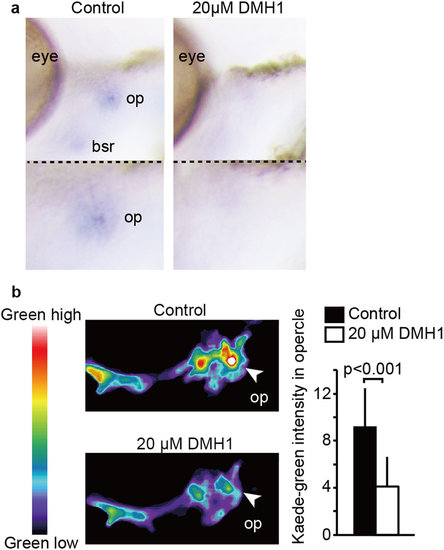
BMP pathway upregulates runx2 expression during opercle formation. (a) Embryos of Tg(fli1: gal4-vp16) were treated with or without 20 µM DMH1 from 54 to 72 hpf, fixed, and WISH was performed to detect the mRNA of runx2a. (b) Tg (runx2enhancer: gal4-vp16): (UAS: kaede) were treated with or without DMH1 from 54 hpf, irradiated with UV at 60 hpf, and Kaede-green intensity in the opercle area was measured at 72 hpf. Representative images are shown with pseudo color. Average and SD of Kaede-green intensity were calculated from more than 22 embryos in each sample, and are shown in right panel. |
|
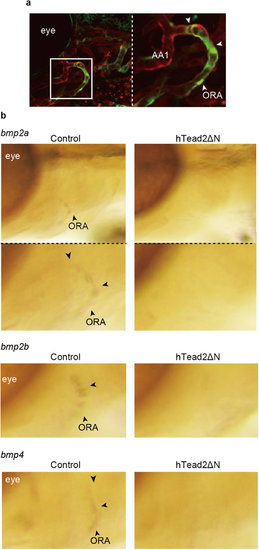
Yap/Taz transcriptional activity is required for bmp expression in ORA. (a) Representative images of ORA in Tg(fli1: gal4-htead2ΔN): (UAS: GFP): (fli1: myr-mCherry) at 72 hpf are shown. Green color represents Yap/Taz transcriptional activity. Red color represents all endothelial cells. (b) Control Tg(fli1: gal4-vp16) and embryos of Tg(fli1: gal4-vp16): (UAS: GFP-htead2ΔN) were fixed with glutaraldehyde at 72 hpf, and WISH was performed to detect mRNA of bmp2a, 2b, and 4. |
|
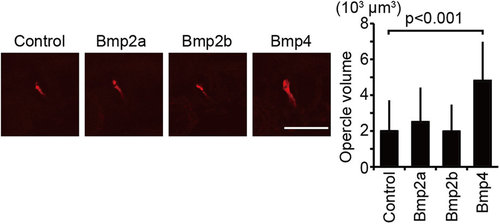
Bmp4 is a target of Yap/Taz during opercle formation. Plasmids containing UAS: bmp2a, UAS: bmp2b, and UAS: bmp4 were introduced to embryos of Tg(fli1: gal4-vp16) at the one-cell stage. Un-injected embryos were prepared as control. Embryos were stained with alizarin red s, and the volume of the opercle (op) at 72 hpf was measured. Average and SD were calculated from more than 18 embryos in each sample. Bar: 50 µm. |
|
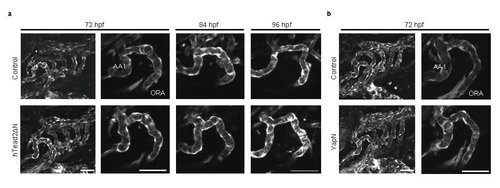
Inhibition of Yap/Taz transcriptional activity in endothelial cells affects little in head angiogenesis, especially in the ORA.(a) and (b) Control embryos, Tg(fli1: gal4-vp16): (UAS: GFP-htead2ΔN), and Tg(fli1: gal4-vp16): (UAS: GFP-hyapN) were crossed with (UAS: mCherry). mCherry expression was visualized in white color as endothelial marker at indicated time. Bar: 50 µm. |
|
The Tg(flk1: gal4-vp16): (UAS: GFP-htead2ΔN) also shows osteogenesis retardation. (a) and (b) Control Tg(flk1: gal4-vp16) embryos and Tg(flk1: gal4-vp16): (UAS: GFP-htead2ΔN) were stained with alizarin red s, and the volume of the opercle (op) and the diameter of the otolith (ot) at indicated hours post fertilization (hpf) were measured. Average and SD were calculated from more than 15 embryos in each sample. Bar: 50 µm. |
|
The Tg(fli1: gal4-vp16) and the Tg(flk1: gal4-vp16) selectively express gal4-vp16 gene in endothelial cells. The Tg(fli1: gal4-vp16) and the Tg(flk1: gal4-vp16) were crossed with Tg(UAS: GFP) and analyzed at indicated hpf. Branchial arteries (AA), opercular artery (ORA), adductor mandibulae (am), levatorarcus palatini (lap), adductor operculae (ao), intermandibularis posterior (imp), interhyoideus (ih), intermandibularis anterior (ima), intermandibularis posterior (imp), and hyohyoideus (hh) are also indicated. Bar: 50 µm. |
|
Inhibition of Yap/Taz transcriptional activity in osteoblast cells affects little in the opercle formation. (a) Stacked images of Tg(osterix: gal4-htead2ΔN): (UAS: GFP) at indicated hpf are shown. Green color represents Yap/Taz transcriptional activity. Bar: 50 µm. (b) and (c) Control embryos of Tg(osterix: gal4-vp16) and Tg(osterix: gal4-vp16): (UAS: GFP-htead2ΔN) were stained with alizarin red s, and measured the volume of opercle (op) at indicated hpf. Average and SD are calculated with more than 5 embryos in each sample. Bar: 50 µm. (d) Representative images of Tg(osterix: gal4-vp16): (UAS: GFP) at indicated hpf are shown. Bar: 50 µm. (e) and (f) Stacked images of Tg(runx2enhancer: gal4-vp16): (UAS: GFP) at indicated hpf are shown. Green color represents GFP expression. In (f), a stacked image in longer exposure is shown. In upper panel, left arrowheads indicate hyaloid vessels. In lower panel, left arrows indicates superficial annular vessel. Right arrowhead indicates hyaloid vessels. Bar: 50 µm. (g) and (h) Similar experiments were performed as (b) and (c) by using Tg(runx2enhancer: gal4-vp16): (UAS: GFP-htead2ΔN). Average and SD are calculated with more than 10 embryos in each sample. Bar: 50 µm. |
|
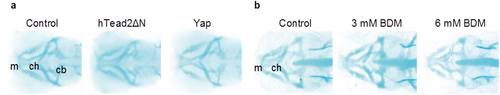
Inhibition of Yap/Taz transcriptional activity in endothelial cells or blood circulation affects little in head endochondral development. (a) Control Tg(fli1: gal4-vp16), Tg(fli1: gal4-vp16): (UAS: GFP-htead2ΔN), and Tg(fli1: gal4-vp16): (UAS: GFP-hyap) were fixed at 72 hpf and stained with alcian blue for detecting cartridge. (b) Similar experiments were performed as (a). Control Tg(fli1: gal4-vp16) were treated with BDM from 54 hpf to 72 hpf. Meckel′s (m), ceratohyal (ch), and ceratobranchial (cb) cartilage are indicated. |