- Title
-
Nkd1 functions as a passive antagonist of wnt signaling
- Authors
- Angonin, D., and Van Raay, T.J.
- Source
- Full text @ PLoS One
|
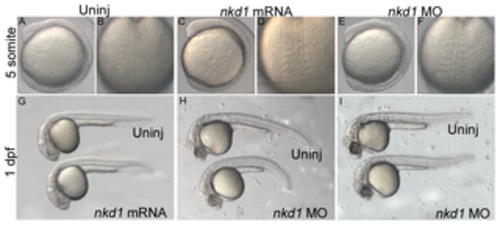
Figure 1. Nkd1 does not influence normal development and patterning of the early embryo. Injection of nkd1 mRNA or morpholino (MO) does not have an obvious affect during early somitogenesis (A–F) or at 1 dpf (G–I). The number and width of somites in injected individuals is indistinguishable from uninjected embryos. At 1 dpf, injection of nkd1 MO results in neural necrosis, which ranges from moderate (H) to more severe (I). |
|
Figure 2. Nkd1 is sufficient to antagonize ectopic Wnt8a. Overexpression of Wnt8a (25pg) results in an eyeless phenotype that can be rescued by co-injection of nkd1 (A, B) which is quantified in (C). Numbers above each column represent n values. Overexpression of high Wnt8a (200pg) results in ectopic gsc expression along the ventral-lateral domain at 50% epiboly (E). Co-injection of high wnt8a with nkd1 mRNAs dramatically reduces the ectopic gsc expression, but leaves the putative endogenous gsc domain intact (F). EXPRESSION / LABELING:
|
|
Figure 3. Nkd1 is insufficient to antagonize constitutively active β-catenin. Overexpression of ΔN-β-catenin results in a spectrum of dorsal-ventral (D-V) phenotypes ranging from a severe phenotype (A), which shows dramatic reduction in both dorsal and ventral structures to a moderate phenotype (B), which have reduced dorsal and ventral structures. The addition of Nkd1 does not ameliorate the effect of &Delat;N-β-catenin. The distribution of phenotypes in ΔN-β-catenin and nkd1 injections is quantified in (D), with numbers above each column representing n values. Consistent with the 1 dpf phenotype, ΔN-β-catenin overexpression results in expansion of gsc expression (E, F), which is not reduced by the addition of Nkd1 (G) (uninj n=40; ΔN-β-catenin n=32; ΔN-β-catenin+nkd1 n=34). EXPRESSION / LABELING:
|
|
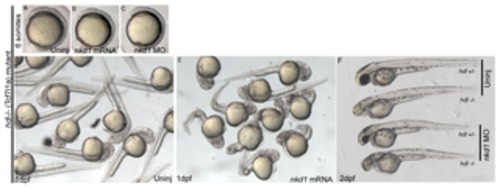
Figure 4. Nkd1 does not rescue the hdl/tcf7l1a mutant. Homozygous deletion of tcf7l1a results in an eyeless phenotype due to activated canonical Wnt signaling (D). Overexpression of Nkd1 in embryos from a homozygous hdl-/- parental cross (B, E) does not rescue the eyeless phenotype (E) and does not affect development of the early embryo (B), although at 1 dpf, nkd1 injected embryos typically have a kinked axis. Injection of nkd1 MO into embryos from a hdl +/- X hdl -/- parental cross has no affect on early (C) or 1 dpf (F) development (Uninj hdl +/- n= 14, hdl -/-n= 16; Nkd1 MO injected hdl +/- n= 15, hdl -/-n=22). Experiments in embryos from homozygous hdl-/- parental crosses had similar results (not shown). PHENOTYPE:
|
|
Figure 5. The silberblick (Wnt11) mutant is sensitive to Nkd1. The slb mutant undergoes normal convergence and extension (A), which is not affected by knockdown of Nkd1 with morpholinos (B). Overexpression of Nkd1 in slb-/- reduces gsc (D) and chd (F) expression (arrows), relative to controls (C, E) at 50% epiboly (Nkd1 injected: 47% of embryos with reduced chd expression (n=81); 50% of embryos with reduced gsc expression (n=46)). In contrast, knockdown of Nkd1 results in a slight expansion of gsc expression at 30% epiboly (G: ave gsc width=0.35 mm; n=13, H: ave gsc width=0.38 mm; n=22). All embryos are homozygous slb, derived from homozygous slb parents. EXPRESSION / LABELING:
|
|
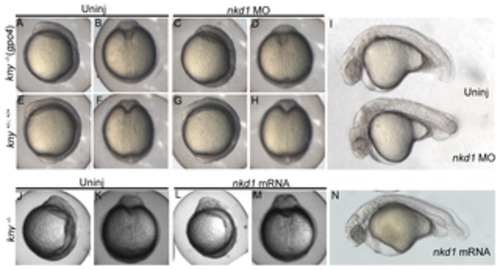
Figure 6. The knypek (glypican 6) mutant is not sensitive to Nkd1. Knockdown of Nkd1 in kny -/- (C, D) or in kny +/+; +/- (G, H) does not have any affect on the kny mutant phenotype during somitogenesis (A–H) or at 1 dpf (I; n=19 kny-/-). Note the high levels of neural necrosis in nkd1 MO injected embryos at 1 dpf (I). Consistent with the lack of sensitivity, overexpression of Nkd1 has no obvious effects during early somitogenesis (J–M) or at 1 dpf (N; n=31 kny -/-). There is no change in the ratio of wild-type: mutant phenotypes. |
|
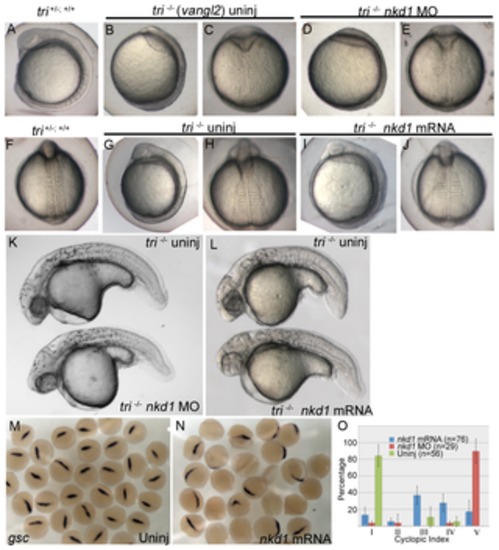
Figure 7. Trilobite (vangl2) mutants are sensitive to Nkd1. Knockdown of Nkd1 with morpholinos (D, E) or overexpression of Nkd1 (I, J) does not have an obvious effect during early somitogenesis. However, at 1 dpf, knockdown or overexpression of Nkd1 in tri mutants results in an increase in cyclopia (K, L, O). Before the onset of gastrulation, at 50% epiboly, embryos generated from a tri+/- X tri+/- parental cross are sensitive to Nkd1 overexpression, demonstrated by a reduction or absence of gsc expression (M; n=32, N; 56% of embryos with reduced expression, n=25). (O) The cyclopic index was calculated using criteria established in Marlow et al., 1998 [43]. n values reflect the number of tri-/- embryos. Error bars represent standard error. PHENOTYPE:
|
|
Figure 8. Trilobite (vangl2) mutants are highly sensitive to canonical signaling. Injection of a low dose (5pg) of wnt8a into embryos from a tri+/- X tri+/- parental cross results in extremely dorsalized embryos and significant lethality (A–D, I). Different classes of phenotypes are shown in (A–D) with an uninjected wild-type sibling shown at the top in (A), and an uninjected tri mutant shown at the top in (B) for comparison. Addition of Nkd1 is capable of fully suppressing the wnt8a overexpression lethality and dorsalization (I). n values are for all genotypes. In contrast to the extreme phenotypes seen at 1 dpf, there is no effect of Wnt8a on the early organizer (E–H) shown by expression of gsc (E, F) and bozozok (boz) (G, H) an early and direct transcription target of maternal Wnt signaling. (J–M) Injection of vangl2 MO does not alter the expression of gsc (K), nor does the low level of wnt8a (M). However, co-injection of vangl2 MO and wnt8a results in ectopic gsc expression about 2-5% of the time (red arrowheads, n= 21). EXPRESSION / LABELING:
|