- Title
-
Thyroid hormone and retinoic acid interact to regulate zebrafish craniofacial neural crest development
- Authors
- Bohnsack, B.L., and Kahana, A.
- Source
- Full text @ Dev. Biol.
|
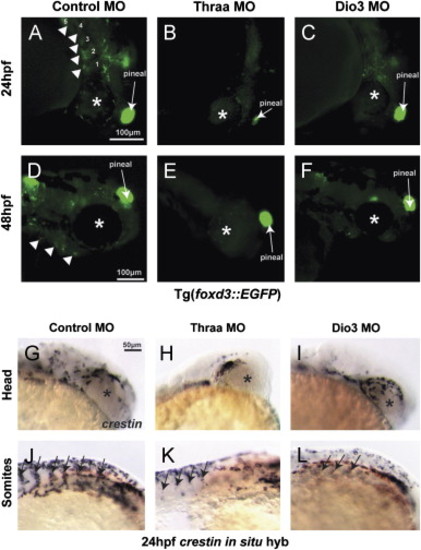
TH regulates early neural crest development. Thraa MO knockdown (decreased TH signaling) inhibited neural crest migration at 24 (B) and 48 hpf (E) vs. control (A, D) in Tg(foxd3::EGFP). Thraa MO knockdown inhibited expression at 24 hpf of crestin in the head (H) and body (K; between somites, arrows) vs. control (G, J). Dio3 MO knockdown (increased TH) in Tg(foxd3::EGFP) embryos inhibited PA (arrowheads) formation at 24 (C vs. A) and 48 hpf (F vs. D). In situ hybridization for crestin demonstrated Dio3 MO knockdown impaired neural crest migration in the head (I) and between somites (L, arrows) at 24 hpf. Asterisk denotes developing eye. Scale bar=50 or 100 µm as indicated. |
|
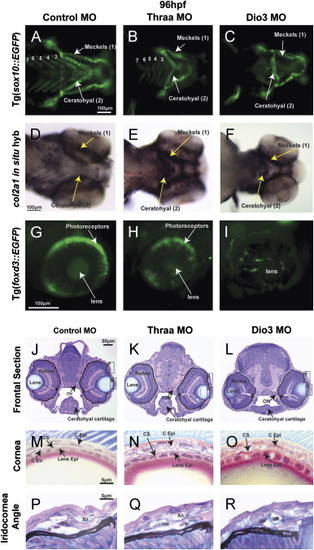
TH is required for craniofacial and ocular development. 96 hpf control embryos demonstrated neural crest-derived structures including 7 PAs (Meckels cartilage (1st PA), ceratohyal cartilage (2nd PA), and 5 posterior PAs) in vivo in Tg(sox10::EGFP) zebrafish (A) and by col2a1 in situ hybridization (D). Control embryos also showed foxd3 expression in photoreceptors at 96 hpf Tg(foxd3::EGFP) embryos (G). Thraa MO knockdown inhibited Meckels and ceratohyal cartilage formation, decreased the number of posterior PAs (B, E), and decreased photoreceptor expression of foxd3 (H). Dio3 MO knockdown inhibited posterior PA formation (C, F) and foxd3 expression in photoreceptors (I). Dio3 MO knockdown had less effect on Meckels and ceratohyal cartilage formation. Methylacrylate sections demonstrated that control embryos (J) had corneas (M) that contained epithelial (C Epi), stromal (C S), and endothelial (C End) layers, lens epithelial cells (Lens Epi) adjacent to the cornea, and iris stroma with xanthophores (Xn), iridophores (Ir), and undifferentiated cells (Un). Thraa MO knockdowns (K, N, Q, T) demonstrated thickened and scalloped C Epi, lack of C End (N) and decreased cellularity of the iridocorneal angle (Q) vs. control (J, M, P). ON, optic nerve; MR, medial rectus. Scale bar=5, 10, 50 or 100 µm as indicated. |
|
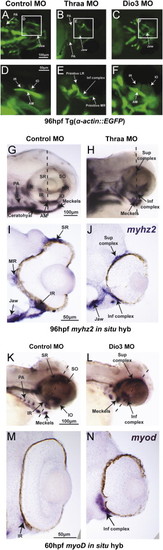
TH regulated craniofacial muscle development including EOM organization. 96 hpf control embryos demonstrated 6 distinct EOM; medial rectus (MR), lateral rectus (LR), superior rectus (SR), superior oblique (SO), inferior rectus (IR), and inferior oblique (IO) in vivo in Tg(α-actin::EGFP) (A, D) and by in situ hybridization for myzh2 (G, I). In situ hybridization for myoD demonstrated early muscle differentiation in EOM at 60 hpf (K, M). Thraa MO knockdown inhibited jaw and PA muscle development (B, H). Thraa MO knockdown did not disrupt muscle differentiation, but caused indistinct superior (sup) and inferior (inf) EOM complexes (E, H, J). Dio3 MO knockdown inhibited posterior PA development (C, L) and caused thickening and overlapping of EOM insertions (F). At 60 hpf EOMs in Dio3 MO knockdowns showed early muscle differentiation (L), but were clustered together and indistinct in wholemount (L) or on sections (N). AM, anterior mandibulae. Scale bar=50 or 100 µm as indicated. |
|
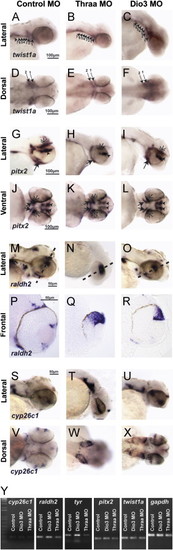
TH regulates expression of twist1a and pitx2. 48 hpf expression of twist1a by in situ hybridization demonstrated decreased expression in Thraa MO knockdowns (B, E) which was expressed in pharyngeal arches (arrowheads) in controls (A, D). Increased TH (via MO knockdown of Dio3) demonstrated increased expression of twist1a within pharyngeal arches (C, F, arrowheads). In situ hybridization for pitx2 demonstrated markedly decreased expression in periocuar mesenchyme of Thraa MO knockdowns (H, K, double arrowheads) and slightly decreased expression in POM of Dio3 (I, L, double arrowheads) MO knockdown. Pitx2 was normally expression in the jaw (arrows), POM (double arrowheads), optic nerve (large arrowheads) and pituitary (small arrowheads) in control embryos (G, J). In situ hybridization demonstrated in control embryos that raldh2 was expressed in ventral and dorsal retina, jaw, and POM at 48 hpf (M, P). In Thraa MO knockdowns, raldh2 was only expressed in the dorsal retina at 48 hpf (N, Q). MO knockdown of Dio3 decreased expression of raldh2 in pharyngeal arches and POM (O, R). In situ hybridization in control embryos demonstrated expression of cyp26c1 in a demarcating line between dorsal and ventral retina, hindbrain, and otic vesicle 48 hpf (S, V). MO knockdown of Thraa (T, W) and Dio3 (U, X) increased expression of cyp26c1 in the retina, hindbrain, and otic vesicle. Scale bar=50 µm. Semi-quantitative RT-PCR of RNA (Y) derived from whole 36 hpf control, Dio3 MO, or Thraa MO demonstrated no difference in expression of cyp26c1, pitx2, and twist1a. RT-PCR demonstrated decreased overall expression of raldh2 and tyrosinase (tyr) in Thraa MO compared to control. Dio3 MO knockdown showed increased expression of tyr. Semi-quantitative RT-PCR used Gapdh and S18 (data not shown) as internal controls. EXPRESSION / LABELING:
|
|
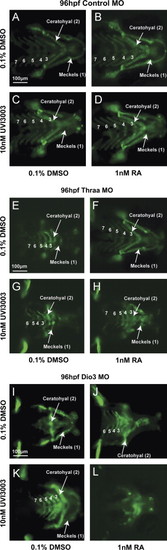
RA and TH have reciprocal interactions on caudal neural crest development that are mediated by RXR. Treatment of Tg(sox10::EGFP) embryos injected with control MO with 1 nM RA starting at 12 hpf had minimal teratogenic effects (B) vs. control media (A). Treatment with RXR antagonist, 10 nM UVI3003 in the absence (C) or presence of 1 nM RA (D) had minimal affect on cranial neural crest development. MO knockdown of Thraa caused malformation of ceratohyal and Meckels cartilage formation (E) which was rescued by 1 nM RA (F) starting at 12 hpf. In contrast, treatment with UVI3003 (10 nM) at 12 hpf further inhibited Meckels, ceratohyal, and posterior PA cartilage (G) and abrogated the effect of exogenous RA (H) on Thraa MO knockdowns. MO knockdown of Dio3 inhibited posterior PA formation (I) which was improved by exogenous RA (1 nM, J) starting at 12 hpf. 10 nM UVI3003 starting at 12 hpf improved posterior pharyngeal arch and ceratohyal cartilage in Dio3 MO knockdowns (K), however, co-treatment with 10 nM UVI3003 and 1 nM RA completely suppressed pharyngeal arch and jaw development (L). Scale bar=100 µm. |
|
|
|
|
|
|
|
|
|
EXPRESSION / LABELING:
PHENOTYPE:
|
|
|
|
|
|
|
Reprinted from Developmental Biology, 373(2), Bohnsack, B.L., and Kahana, A., Thyroid hormone and retinoic acid interact to regulate zebrafish craniofacial neural crest development, 300-309, Copyright (2013) with permission from Elsevier. Full text @ Dev. Biol.