- Title
-
NAD+ biosynthesis ameliorates a zebrafish model of muscular dystrophy
- Authors
- Goody, M.F., Kelly, M.W., Reynolds, C.J., Khalil, A., Crawford, B.D., and Henry, C.A.
- Source
- Full text @ PLoS Biol.
|
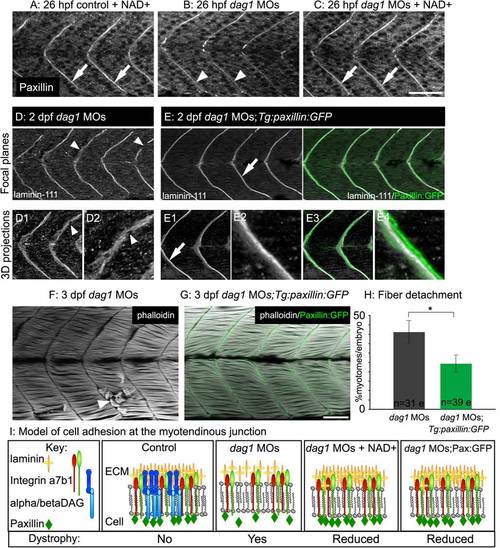
Exogenous NAD+ improves the structure of muscle in dag1 morphants. (A-B) Anterior left, dorsal top, side-mounted 2 dpf embryos stained for actin (phalloidin, red) and laminin-111 (green or white). Qualitatively, laminin-111 antibody staining appears to be within myotomes and less well aligned at the MTJ BM in dag1 morphants (A) compared to dag1 morphants treated with 0.1 mM NAD+ (B). White boxes in (A) and (B) correspond to numbered panels below. White arrowheads indicate holes in the MTJ BM. (A3, B3) 2DWTMM analysis of laminin-111 stained dag1 morphants (A3) and NAD+-supplemented dag1 morphants (B3). Maxima nodes are in red, maxima chains are in blue, and vectors pointing in the direction of the maximum intensity gradient are in green. Parallel vectors reflect greater organization. (C) Quantification of the anisotropy factor. The anisotropy factor is the sum of the vector angles. A greater anisotropy factor denotes more organization. NAD+ treatment of dag1 morphants (blue bars) significantly increases organization of laminin-111 compared to dag1 morphants (gray bars) over multiple size scales; *p<0.05, **p<0.01. (D) Model of the MTJ. Transmembrane receptors, integrins, and the DGC bind extracellular laminin. In dag1 morphants, laminin is less organized at the MTJ BM. Exogenous NAD+ improves laminin organization in the MTJ BM in Dag1-deficient zebrafish. Scale bar is 50 micrometers. |
|
An organized ECM microenvironment rescues fiber resiliency in Dag1-deficient cells. (A) Anterior left, dorsal top, side-mounted, 3 dpf embryos. Polarized light microscopy shows loss of birefringence in dag1 morphant myotomes (white arrowheads). Birefringence is rescued in NAD+-supplemented dag1 morphants. (B, C, I, J, L) Anterior left, dorsal top, side-mounted, 3 dpf embryos stained with phalloidin (white or green). Fiber detachment is readily observed in dag1 morphants (B, white arrowheads), whereas dag1 morphants supplemented with NAD+ display less fiber detachment (C). (D) Compared to dag1 morphants (gray bars), NAD+ supplementation (blue bar) and vitamin supplementation with Emergen-C (purple bar) significantly reduce fiber detachment; **p<0.01. (E-H) Transmission electron micrographs showing normal BMs (white arrows) and disrupted BMs (red arrows). (I) The dystrophic phenotype of 3 dpf sly/laminin gamma1 mutant zebrafish. White arrowhead points to detached fibers. (J) NAD+ does not rescue the dystrophic phenotype in sly mutants, suggesting that NAD+-mediated amelioration of dystrophy requires laminin. (K) Genetic mosaic cartoon depicting transplantation of fluorescent dextran-labeled dag1 morphant (red) and control (blue) cells into unlabeled, control hosts. Some embryos were stressed (frequently stimulated to swim in a viscous medium), and all hosts were reared to 3 dpf. (L) Transplanted control cells (blue) and dag1 morphant cells (red) remain attached to MTJs, even when hosts are stressed. This suggests that a normal host ECM microenvironment is sufficient for resiliency of dag1 morphant cells and supports that NAD+ functions via augmentation of the ECM microenvironment. (M) The vast majority of Dag1-deficient cells remain attached in unstressed (513/523 Dag1-deficient cells were attached) and stressed hosts (479/491 Dag1-deficient cells were attached), N.S., not significant. Scale bars are 50 micrometers in (B) and (J) and 5 micrometers in (E-H). PHENOTYPE:
|
|
Itga7 is required for NAD+-mediated reduction of fiber degeneration in dag1 morphants. (A-D, G–H) Anterior left, dorsal top, side-mounted embryos stained with phalloidin (red or white) or pY397 FAK (green). (A-B) Muscle morphogenesis proceeds normally in itga7 morphants. Phosphorylated FAK (green) outlines fibers and concentrates at the MTJ, and actin distribution in slow- and fast-twitch muscle fibers (red) appears normal in 26 hpf itga7 morphants compared to wild-types. (C) 3 dpf dag1;itga7 double morphant. (D) 3 dpf NAD+-supplemented dag1;itga7 double morphant. MTJ morphogenesis is disrupted in dag1;itga7 double morphants as displayed by wider MTJ angles (C). MTJ morphogenetic defects were rescued by NAD+ in dag1;itga7 double morphants (D), suggesting that another laminin receptor is sufficient for NAD+-mediated MTJ improvements. (E) Quantification of MTJ angles shows that dag1;itga7 double morphants have significantly wider MTJ angles than wild-types, and NAD+ significantly reduces this defect; **p<0.01, *p<0.05. (F) Quantification of incidence of dystrophy per embryo shows no significant difference in dag1;itga7 double morphants upon addition of exogenous NAD+, suggesting that Itga7 is required for NAD+-mediated reduction of dystrophy in dag1 morphants; N.S., not significant. However, fewer fibers appeared to detach in dag1;itga7 double morphants supplemented with NAD+ (D), again suggesting the involvement of another receptor for laminin in NAD+ action. (G–H) Mild fiber detachment is readily observed in 4 dpf itga7 morphants (G) and reduced in NAD+-treated itga7 morphants (H). (I) NAD+ treatment significantly decreases fiber degeneration in itga7 morphants; ***p<0.001. (J) Model of cell adhesion at the MTJ. The transition from a somite boundary to a MTJ involves the downregulation of Fibronectin and the upregulation of laminin and laminin receptors. Our results suggest that laminin receptors, Itga7 and Dag1, play a role in this transition, but the primary receptor involved is an unknown integrin. In maintenance of fiber adhesion at the MTJ, our data show that either Itga7 or Dag1 is required, but also suggest the involvement of an additional laminin receptor. Scale bar is 50 micrometers. |
|
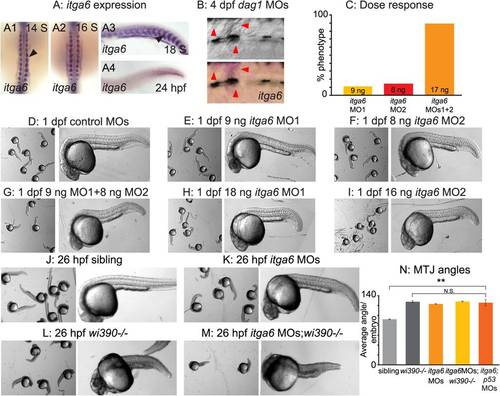
itga6 is upregulated in regenerating muscle and characterization of itga6 MOs. (A) In situ hybridizations showing itga6 expression (purple). (A1–2) Dorsal view, anterior top. (A3–4, B) Side view, anterior left, dorsal top. (A1-4) Black arrowheads denote somitic expression. itga6 expression is high during early muscle development, then decreases. (B) itga6 is re-expressed in regenerating muscle. itga6, not normally expressed in muscle at 4 dpf, is observed in dystrophic lesions of 4 dpf dag1 morphants (red arrowheads). (C) itga6 MO characterization. Dose response graph. MO1 and MO2 generate the same phenotype and synergize when co-injected. (D–M) Brightfield images, side view, anterior left, dorsal top, 1 dpf embryos. (D-I) Phenotypic analysis of itga6 MOs 1 and 2. Embryos injected with low doses of MO1 (E) or MO2 (F) are morphologically similar to controls (D). Combining the two lower doses of MOs 1 and 2 results in a truncated body axis with myotomes that are narrower in the anterior-posterior dimension (G). The identical phenotype is obtained when higher doses of either MO1 (H) or MO2 (I) are injected. (J-M) Pseudo-genetic epistasis analysis. (J) Siblings, (K) itga6 morphants, (L) wi390-/-/laminin gamma1 mutants, and (M) itga6 MOs;wi390-/-. Note that injection of itga6 MOs into laminin mutants does not change their phenotype, suggesting Itga6 functions in laminin signaling and adhesion. (N) Average MTJ angles of 1 dpf embryos. MTJ angles in morphants, mutants, and morphant/mutants do not significantly differ from one another and are all significantly wider than in sibling controls; **p<0.01; N.S., not significant. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Itga6 functions in the Nrk2b-NAD+-laminin pathway and is required for NAD+-mediated rescue of MTJ morphogenesis and dystrophy. (A-C, E-F, H-I) Anterior left, dorsal top, side-mounted, 2 dpf embryos stained with phalloidin (white) to visualize actin. In pseudocolored panels (A1, B1, C, E, F), MTJ boundaries are blue, and abnormally long muscle fibers are red. (A) MTJs are V-shaped and continuous in control embryos. (B) In itga6 morphants, MTJs are U-shaped, discontinuous, and crossed by abnormally long muscle fibers (red arrows). (C) Co-injection of p53 MOs does not rescue MTJ failure in itga6 morphants. (D) Quantification of MTJ failure at 2 dpf in controls, itga6 morphants, and itga6;p53 double morphants. (E–F) Co-injection of itga6 cDNA that does not contain the MO target sites with itga6 MOs rescues the itga6 morphant phenotype. (G) Quantification of MTJ failure shows that NAD+ treatment does not rescue MTJ failure in itga6 morphants, suggesting that NAD+ requires Itga6 for rescue of MTJ failure. (H) dag1;itga6 double morphants have U-shaped MTJs and dystrophy (white arrowheads). (I) NAD+ does not reduce MTJ angles (not shown) or dystrophy in dag1;itga6 double morphants, suggesting that Itga6 is also required for NAD+-mediated rescue of MTJ angles and dystrophy. (J) Quantification of dystrophy shows significant rescue by exogenous NAD+ in dag1 morphants, but not dag1;itga6 double morphants; *p<0.05; N.S., not significant. Scale bars are 50 micrometers. PHENOTYPE:
|
|
Paxillin overexpression increases laminin organization and ameliorates dystrophy in dag1 morphants. (A–C) Anterior left, dorsal top, side-mounted, 1 dpf embryos antibody stained for paxillin (white). (A) Paxillin concentrates at the MTJ in both untreated (not shown) and NAD+-treated controls (white arrows). (B) Paxillin is less concentrated at the MTJ in dag1 morphants (white arrowheads). (C) NAD+ rescues the disrupted concentration of paxillin at the MTJ in dag1 morphants (white arrows). (D-E) Anterior left, dorsal top, side-mounted, 2 dpf embryos stained with laminin-111 antibody (white). Numbered panels are 3-D reconstructions. (D) dag1 morphant laminin-111 appears within myotomes, and the MTJ BM is poorly aligned medially laterally and contains holes (white arrowheads). (E) In contrast, paxillin overexpression (green) in dag1 morphants reduces laminin-111 within myotomes and enhances organization of laminin-111 at the MTJ BM (white arrows). (F-G) Anterior left, dorsal top, side-mounted, 3 dpf embryos stained for actin (phalloidin, white). (F) dag1 morphant with detached fibers (white arrowhead). (G) Transgenic overexpression of paxillin (green) in dag1 morphants reduces fiber detachment. (H) Paxillin overexpression significantly reduces the frequency of fiber detachment in dag1 morphants; *p<0.05. (I) Model of cell adhesion at the MTJ in response to Nrk2b pathway activation via exogenous NAD+ or paxillin overexpression. Scale bars are 50 micrometers. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Paxillin action but not subcellular localization requires functional integrin receptors for laminin. (A–B, D–E) Side-mounted, anterior left, dorsal top, 2 dpf embryos stained with phalloidin (white). (A–B) Antibody staining shows that paxillin (green) concentrates at the MTJ in itga6 morphants (B) as in controls (A). (C) Quantification of MTJ failure shows that paxillin overexpression does not rescue itga6 morphants. (D-E) Transgenic overexpression of paxillin:GFP (green) does not affect MTJ development in controls (D) and is not sufficient to rescue MTJ failure in itga6 morphants (E). (F-G) Anterior left, dorsal top, side-mounted, 26 hpf embryos stained for paxillin (white). Paxillin concentrates at the MTJ in itga7 morphants (G) as in controls (F). (H-I) Side mounted, anterior left, dorsal top, 4 dpf embryos stained with phalloidin (white). Fiber detachment is readily observed in itga7 morphants (H) and itga7 morphants transgenically overexpressing paxillin (I, white arrowheads). (J) Paxillin overexpression does not affect fiber detachment frequency in itga7 morphants. Together, these results suggest that Itga6 and Itga7 are required for paxillin-mediated improvements in muscle tissue. N.S., not significant. Scale bars are 50 micrometers. |
|
NAD+ supplementation, but not paxillin overexpression, improves motility of dystrophic zebrafish. (A-F) Individual panels from videos of escape responses after a touch stimulus at 2 dpf; time in milliseconds is denoted on panels. The outer circle is 10 mm in diameter. Red arrowheads point to the embryo′s location. (A) Control embryo. (B) dag1 morphant. (C) NAD+-supplemented dag1 morphant. (D) itga7 morphant. (E) NAD+-supplemented itga7 morphant. (F) dag1 MOs;Tg:paxillin:GFP embryo. (G) Average escape response times of 2 dpf dystrophic zebrafish after exogenous NAD+ treatment or overexpression of paxillin. Exogenous NAD+ or Emergen-C (not shown) significantly reduced the escape times of both dag1 and itga7 morphants. Overexpression of paxillin, however, did not reduce escape times of dag1 or itga7 morphants. *p<0.05; **p<0.01; ***p<0.001; N.S., not significant. (H) Model of cell adhesion at the MTJ. Our data show that laminin polymerization is necessary and sufficient for muscle fiber homeostasis and that NAD+ and paxillin increase laminin polymerization. We find that Dag1 and Nrk2b are required for paxillin localization to the MTJ. We hypothesize that NAD+, through mediating paxillin concentration at MTJs, invokes “inside-out” signaling through laminin receptors that results in increased laminin polymerization. PHENOTYPE:
|