- Title
-
Role of Chd7 in Zebrafish: A Model for CHARGE Syndrome
- Authors
- Patten, S.A., Jacobs-McDaniels, N.L., Zaouter, C., Drapeau, P., Albertson, R.C., and Moldovan, F.
- Source
- Full text @ PLoS One
|
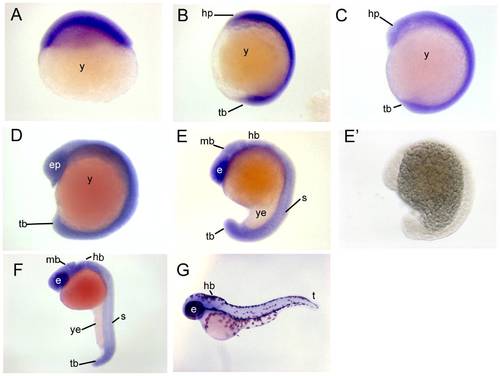
Chd7 expression patterns during zebrafish embryogenesis. Chd7 was expressed ubiquitously throughout the embryo during epiboly (A), at the 4 somite stage (B), and at the 8 somite stage (C). (D) At the 13 somite stage, expression remained relatively ubiquitous, but with stronger expression noted within the retina and at the perimeters of developed somites. (E) At the 18 somite stage, tissue-specific expression was observed in the eyes, brain, somites, and tailbud. This expression pattern remained through 24 hours post fertilization (F). (E2) No staining occurred when hybridized with a chd7 sense probe. (G) At 48 hours post fertilization, chd7 expression began to diminish from the body of the zebrafish but remained in the eye and in the brain. e, eye; ep, eye primordium; mb, midbrain; hp, head primordium; s, somite; t, tail; tb, tailbud; y, yolk; ye, yolk extension. EXPRESSION / LABELING:
|
|
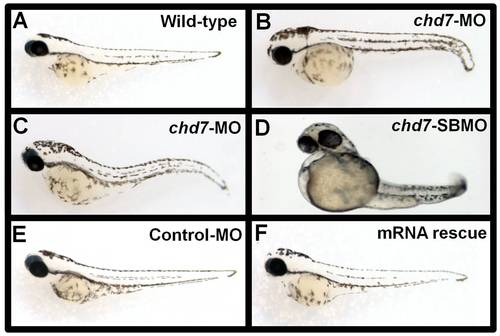
Chd7- MO injections and mRNA rescue experiments. Control-MO injected (E) chd7-MO+chd7-mRNA co-injected (F) zebrafish showed no phenotypic defects at 48 hpf and were comparable to wild type zebrafish (A) at the same age. Embryos injected with 2 ng/nl chd7-MO (B–C), or 2 ng/nl (D) chd7-SBMO showed several developmental defects. PHENOTYPE:
|
|
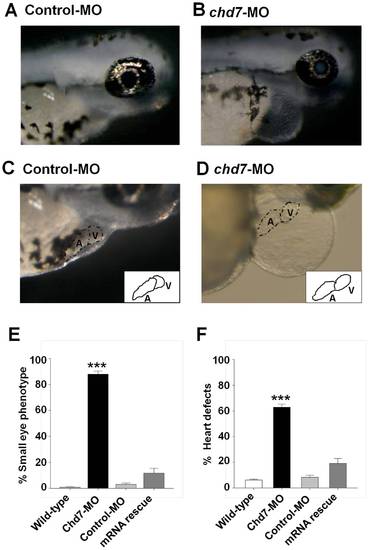
Knockdown of chd7 affects eye and heart development. (A–B) Representative images of eye phenotypes induced by injection of chd7-MO in zebrafish embryos (B) compared with control embryos (A) at 72 hpf. Lateral view with anterior to right. Chd7 morphants had a reduced ocular size. Chd7-MO-injected zebrafish exhibited cardiac anomalies such as dysmorphic heart and tube-like heart shape (D) compared with control embryos (C). Insets in (C) and (D) represent an illustration of the heart phenotype in control or chd7 morphant embryos, respectively. (E–F) Bar graphs illustrating the prevalence of eye (E) and heart (F) phenotypes in wild-type (white bar), chd7-MO (black bar), control-MO-injected embryos (grey bar) and mRNA rescue (dark grey bar). ***<0.001, *<0.01. ***<0.001, *<0.01. PHENOTYPE:
|
|
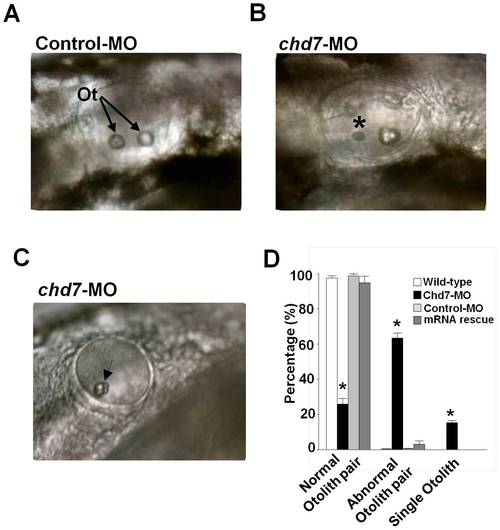
Knockdown of chd7 affects otolith development. (A) The otic vesicle of control-MO-injected fish contains two otoliths, by contrast, larva treated with a morpholino against chd7 (B,C) had smaller otoliths (*) or only a single otolith (Arrow). Lateral views with anterior to the left. Ot, otoliths (D) Bar graphs illustrating the prevalence of otolith phenotypes in wild-type (white bar), chd7-MO (black bar), control-MO-injected embryos (grey bar) and mRNA rescue (dark grey bar). ***<0.001, *<0.01. PHENOTYPE:
|
|
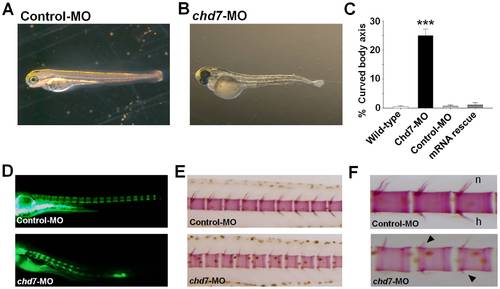
Chd7 deficiency affects vertebral mineralization. Lateral view of control-MO (A) and chd7-MO-injected (B) fish at 3 dpf. Chd7 morphants exhibited a slight curvature of the long body axis (B). A significant percentage of the chd7-MO-injected (2 ng/nl) fish had deformed body axis shape (C). Lateral views of chd7 morphants (lower panel) and control siblings (upper panel) at 8 dpf (D) and 14 dpf (E) showed a reduced bone mineralization. Vertebrae with reduced or no hemal or neural spines were also observed in the chd7-MO-injected fish (F; black arrow). h, hemal spine; n, neural spine. ***<0.001. PHENOTYPE:
|
|
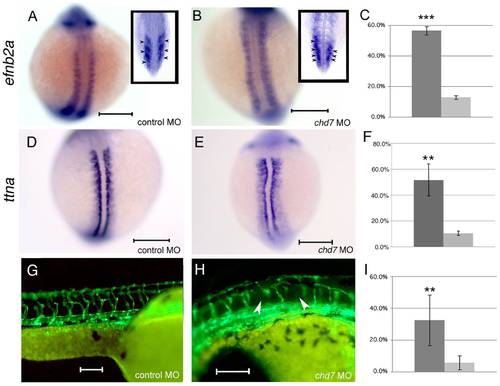
Chd7 knockdown leads to somite boundary and segmental vasculature defects. Efnb2a expression in control morphant (A) and chd7 morphant (B) zebrafish embryos at the 13-somite stage of development. Top is anterior. Insets in (A) and (B) represent coronal sections through control or chd7 morphant embryos, respectively, at the 13 somite stage. Posterior views with dorsal towards the top. Expression of ttna, a segment boundary marker reiterates the defects in segment boundary formation in chd7 morphants (E) compared to controls (D). Dorsal views with anterior to the top. Segmental vasculature patterning is defective (arrowheads) in chd7 morphants (H) compared to control morphants (G) as visualized using fli1: GFP transgenic zebrafish at 48 hpf. Sagittal views with anterior to the right. Graphs show percentages of animals exhibiting efnb2a expression defects (C), ttna defects (F), or segmental vasculature patterning defects (I). The dark gray bar represents chd7 morphants and the light gray bar represents control morphants. Scale bar: 200 μm. ***<0.001, **<0.01. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Chd7 plays an essential role in retinal development. Retinal organization of control-MO embryos (A) and chd7 morphants (B) was revealed by toludiene blue staining. Compared with the highly organized cells and laminated retinal structure in control-MO fish, chd7-MO retinal cells are disorganized (A–B: Left panels). Retinal lamination defect, including rosette formation,is clearly visible in the chd7 morphants (examples of rosettes are indicated by dotted lines and arrow heads). GL, ganglion cell layer; INL, inner nuclear layer; L, lens; ONL, outer nuclear layer; R, retina; RPE, retinal pigment epithelium. Scale bar: 50 μm. Zn-8 immunoreactivity was performed to label (brown) retinal gangion cells in control-MO (C) and chd7-MO embryos (D). Scale bar: 30 μm. The expression of retinal ganglion cell-specific marker zn-8 is greatly reduced in chd7 morphants. The photoreceptor layer of control-MO (E) and chd7-MO-injected (F) embryos were stained with 3A10. Chd7 morphants lacked the photoreceptor layer. Scale bar: 5 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
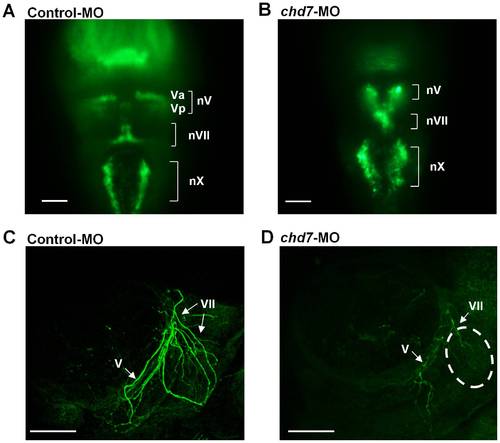
Chd7 is important for cranial ganglia development and proper projection of facial nerves. Dorsal view (rostal towards the top) of confocal fluorescent composite images of hindbrain branchiomotor neurons in control-MO injected (A) and chd7-MO-injected (B) 48 hpf Isl1-GFP transgenic embryos. (C, D) Confocal fluorescent composite images showing anti-3A10 antibody-stained axons of 48 hpf embryos. The broken lines indicate the area of significant loss of VII nerves in chd7 morphants. nV, trigeminal motoneurons; Va, anterior trigeminal motoneurons; Vp, posterior trigeminal motoneurons; nVII, facial motoneurons; nX, Vagal motoneurons; V, trigeminal nerve; VII, facial nerve. Scale bar: 50 μm. |
|
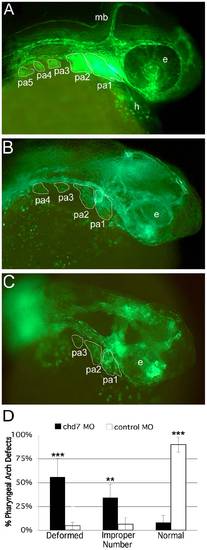
Knockdown of chd7 results in defects in CNC development. Fli1-GFP transgenic embryos were used for MO injections to determine whether Chd7 depletion had an effect on CNC development. Embryos were injected at the 1 cell stage and examined for effects at 34–36 hpf. (A) Control-MO injected zebrafish embryos did not exhibit defects in CNC segment shape or number. Alternatively, the majority of chd7-MO-injected embryos exhibited aberrant CNC development including an improper number of CNC segments (B), as well as severely disorganized (C) or missing CNC populations. (D) Based on the t-test comparing means, there was a significant difference in CNC defects between chd7-MO and control-MO-injected zebrafish embryos. More than half of the chd7-MO-injected fish had deformed or missing CNC segments and about one third developed an improper number of segments. ** = 0.01; ***<0.001; e, eye; mb, midbrain; pa1–5, pharyngeal arches 1–5. |
|
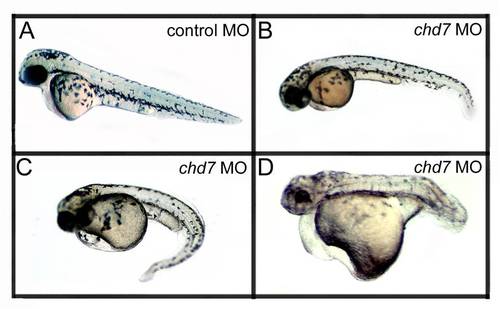
Chd7- MO injections are dosage dependent. As the concentration of the chd7-MO injection increased, the phenotypic defects became more severe. (A) Control-MO injected zebrafish showed no phenotypic defects 48 hpf and were comparable to wild type zebrafish at the same age. Embryos injected with 2 ng/nl (B), 4 ng/nl (C), or 6 ng/nl (D) chd7-MO showed increasing severity of developmental defects with increasing MO concentration. |
|
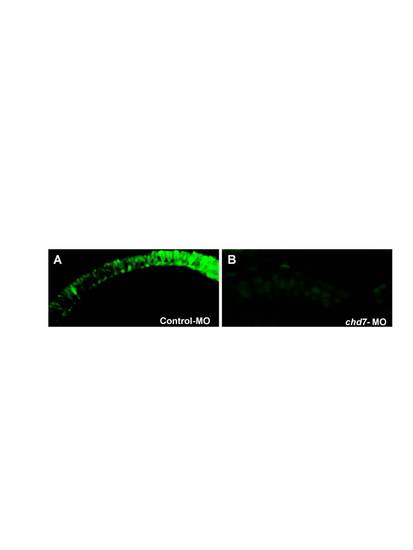
Chd7 plays an essential role in photoreceptor development. The photoreceptor layer of control-MO (A) and chd7-MO-injected (B) embryos were stained with Zpr-1. Chd7 morphants lacked the photoreceptor layer. EXPRESSION / LABELING:
PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|