- Title
-
Netrin Signaling Breaks the Equivalence between Two Identified Zebrafish Motoneurons Revealing a New Role of Intermediate Targets
- Authors
- Hale, L.A., Fowler, D.K., and Eisen, J.S.
- Source
- Full text @ PLoS One
|
Ntn1a expressed in the muscle pioneers is a candidate signal for stopping VaP axon outgrowth. A. Schematized view of one spinal hemisegment including spinal cord (sc) and overlying muscle. CaP (red) and VaP (green) axons contact muscle pioneers (blue) at the first intermediate target. The VaP axon stalls at the muscle pioneers while the CaP axon continues into ventral myotome (vm). ntn1a mRNA (yellow) is expressed in muscle pioneers [16]. This image and following images are oriented laterally with rostral to the left. B–C. Projected confocal stacks of (B) uninjected and (C) ntn1a SBMO-injected embryos labeled with F59, a slow muscle fiber marker [70]. At 24 hpf, muscle pioneers (green fibers in the middle of the images) are present in both control and MO-injected embryos. D. RT-PCR results confirming uninjected embryos have wildtype ntn1a (450 bp band) that is absent from embryos injected with 5.6 ng of ntn1a SBMO (star). Multiple samples were run in this gel. Lanes containing samples unrelated to ntn1a were cropped from the figure. Scale bar = 20 μm. EXPRESSION / LABELING:
|
|
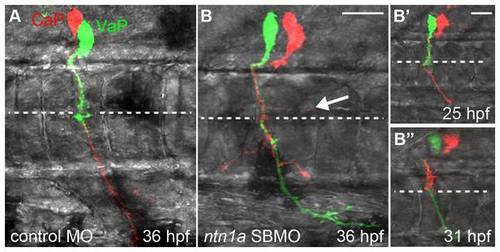
Ntn1a is necessary to prevent a second CaP/VaP axon from extending beyond the muscle pioneers. Projected confocal stacks of living dye-labeled motoneurons in one spinal hemisegment. A. VaP axon stalled at muscle pioneers (dashed line) at 36 hpf in standard control-MO injected embryo. B. nt1a SBMO injected embryos had two CaP/VaP axons that extended beyond the muscle pioneers. One CaP/VaP cell also extended an ectopic branch (arrow) along the horizontal myoseptum. B′-B′′. The same cells as shown in B. At 25 hpf (B′) the red cell was a CaP, but by 31 hpf (B′′) the red cell had retracted its axon to the muscle pioneers intermediate target and the green cell had become CaP. Scale bars = 9 μm in A, B and 20 μm in B′, B′′. |
|
dscam mRNA is expressed in CaPs and VaPs and wildtype transcripts were knocked down with SBMOs. A. A projected confocal stack of dscam mRNA (red) and isl2a mRNA (a CaP/VaP motoneuron marker [41]) expression (green), reveals that at 24 hpf dscam is expressed in both CaP and VaP (*; CaP and VaP pair to left, single CaP to right) as well as other cells that are likely motoneurons. B. RT-PCR results confirming uninjected embryos have wildtype dscam mRNA (395 bp band) while MO-injected embryos have increased dscam pre-mRNA (490 bp band). Starred lanes show dscam transcripts are absent after injecting 8.4 ng of dscam SBMO. Scale bar = 20 μm. EXPRESSION / LABELING:
|
|
Dcc is necessary to prevent a second CaP/VaP axon from extending beyond the muscle pioneers. A. Brightfield image of lateral view of 24 hpf embryo focusing on the spinal cord. dcc mRNA (blue) is co-localized with a motoneuron marker, PARG:GFP (brown; see also [47]). The left segment shows one CaP (*) expressing dcc. The right segment shows two CaP/VaPs (**) expressing dcc. dcc is also expressed in additional cells that are likely other motoneurons and neighboring interneurons. B–D. Projected confocal stacks of living dye-labeled motoneurons in the same dcc TBMO-injected embryo at three developmental stages. Punctate red and green fluorescence within the spinal cord but not in CaP or VaP in this and other figures represents dye leakage from the labeling procedure. The left segment shows a CaP (red) in a segment lacking VaP; the right segment shows both CaP (red) and VaP (green). B. At 26 hpf the VaP axon does not extend beyond the level of the muscle pioneers (dashed line) but CaP axons extend farther. C. By 30 hpf, CaP axons have extended farther ventrally but the VaP axon still remains near the muscle pioneers. D. By 36 hpf, CaP axons have reached the ventral aspect of the myotome, providing evidence that Dcc is unnecessary for CaP axon extension. The VaP axon has extended branches beyond and along the muscle pioneers (arrow). Scale bar = 20 μm. |
|
Dcc is necessary to prevent a second CaP/VaP axon from extending beyond the muscle pioneers. A. Brightfield image of lateral view of 24 hpf embryo focusing on the spinal cord. dcc mRNA (blue) is co-localized with a motoneuron marker, PARG:GFP (brown; see also [47]). The left segment shows one CaP (*) expressing dcc. The right segment shows two CaP/VaPs (**) expressing dcc. dcc is also expressed in additional cells that are likely other motoneurons and neighboring interneurons. B-D. Projected confocal stacks of living dye-labeled motoneurons in the same dcc TBMO-injected embryo at three developmental stages. Punctate red and green fluorescence within the spinal cord but not in CaP or VaP in this and other figures represents dye leakage from the labeling procedure. The left segment shows a CaP (red) in a segment lacking VaP; the right segment shows both CaP (red) and VaP (green). B. At 26 hpf the VaP axon does not extend beyond the level of the muscle pioneers (dashed line) but CaP axons extend farther. C. By 30 hpf, CaP axons have extended farther ventrally but the VaP axon still remains near the muscle pioneers. D. By 36 hpf, CaP axons have reached the ventral aspect of the myotome, providing evidence that Dcc is unnecessary for CaP axon extension. The VaP axon has extended branches beyond and along the muscle pioneers (arrow). Scale bar = 20 µm. |
|
Netrin mRNAs are expressed in intermediate targets of the CaP axon and wildtype transcripts were knocked down with SBMOs. A. Projected confocal stack of ntn1a and ntn1b mRNA expression in zebrafish trunk at 24 hpf; ntn1a is expressed in spinal cord (purple) and muscle pioneers (purple and *). ntn1a staining was false colored purple. ntn1b mRNA (green) is expressed in floor plate (white dots) and hypochord, located ventrally to the notochord. B. Brightfield image of lateral surface of zebrafish trunk at 24 hpf reveals ntn2 is expressed in ventral somite (white arrowheads). Asterisks denote muscle pioneers, cells that do not express ntn2. C. RT-PCR results confirming uninjected embryos have wildtype ntn1b (500 bp band) and ntn2 (300 bp band) transcript. Starred lanes show netrin transcripts are reduced or absent after injecting 10.0 ng of ntn1b SBMO or 5.0 ng of ntn2 SBMO. Multiple samples were run in the gels for ntn1b and ntn2. Lanes containing samples unrelated to the netrins were cropped from the figure. Scale bar = 20 μm. EXPRESSION / LABELING:
|
|
Netrins are unnecessary for CaP axons to extend to intermediate targets. Projected confocal stacks of GFP-expressing motor axons in living Tg(mnx1:GFP) embryos injected with various MOs. A–C. In standard control MO-injected embryos CaP axons extend to the second intermediate target by 24 hpf (A), the third intermediate target by 30 hpf (B), and by 36 hpf have extended past the third intermediate target and wrap around the ventral aspect of the myotome (C). In ntn1a SBMO (D–F), ntn1b SBMO (G–I), and ntn2 SBMO (J–L) injected embryos the time course of CaP axon outgrowth was the same as in controls (A–C). Scale bar = 20 μm. PHENOTYPE:
|
|
Netrins can prevent extension of a second CaP axon into ventral muscle. Projected confocal stacks of living dye-labeled motoneurons in embryos injected with various combinations of MOs. A, D, G, J. Cartoons of a trunk spinal hemisegment detailing ntn1a, ntn1b, and ntn2 mRNA expression. The red X indicates which netrin transcript was knocked down in adjacent panels. B–C. ntn1b SBMO-injected Tg(mnx1:GFP) embryo showing motoneurons (green) and rhodamine dextran-labeled VaPs (yellow). At 24 (B) and 36 hpf (C) VaP axons are stopped at muscle pioneers (upper dashed line). E, F, H, I, K, L. Embryos with individually-labeled CaP and VaP pairs. Dashed ellipses outline motoneuron cell bodies. In some cases interneurons (*) were also injected with dye during the labeling procedure. E–F. In ntn2 SBMO-injected embryos the VaP axon (green) stalled at the muscle pioneers while the CaP axon (red) extended into ventral muscle at 24 hpf (E) and at 36 hpf (F). H–I. In ntn1a plus ntn1b SBMO-injected embryos two axons extended into ventral muscle at 24 hpf (H), but by 36 hpf (I) one axon retracted to the level of the second intermediate target (lower dashed line), becoming CaP-like (green). K–L. In the absence of ntn1a and ntn2 only one axon (green) initially extended to the third intermediate target (K). However, by 36 hpf a second axon (red) extended to the third intermediate target, thus both cells become CaPs (L). Dashed ellipses in L outline the motoneuron cell bodies. Scale bar = 20 μm. |
|
A CaP/VaP-derived signal is not required for expression of the muscle pioneer-derived Netrin signal. This image shows an 18 hpf embryo from which CaP/VaPs were removed before axogenesis from the two segments right of the dotted line, whereas CaP/VaPs were not removed from the segment left of the dotted line. The zn1 and znp1 antibodies used to label the motoneuron axons recognize both motoneuron somata and the somata of some other neurons in the ventral spinal cord [66], [71]. The segment on the left shows a CaP/VaP soma (dot) and its axon (arrow) labeled by the antibodies. The two segments on the right show somata of other neurons, but no CaP/VaP somata or motor axons. ntn1a mRNA (blue) was expressed in the muscle pioneers (*) whether CaP/VaPs were present (left) or absent (right). Scale bar = 20 μm. |