- Title
-
Essential role for fibrillin-2 in zebrafish notochord and vascular morphogenesis
- Authors
- Gansner, J.M., Madsen, E.C., Mecham, R.P., and Gitlin, J.D.
- Source
- Full text @ Dev. Dyn.
|
The pfdgw1 mutation disrupts notochord and vascular development. A: The notochord (black arrow), caudal vein (white arrow), and fin fold (black arrowheads) form normally in wild-type embryos, and melanin pigmentation is present (white arrowhead). B: pfdgw1 mutants exhibit notochord kinking (black arrow), a cavernous caudal vein with loss of the usual reticular venous plexus (white arrow), and fin fold attenuation (black arrowheads). Melanin pigmentation is present (white arrowhead). C: Fin fold (arrowheads) and caudal vein (arrow) in a wild-type embryo. D: Attenuated fin fold (arrowheads) and cavernous caudal vein (arrow) typical of pfdgw1 mutants. E,F: Ventral views of a wild-type embryo (E) and a pfdgw1 mutant (F) demonstrating skin distention secondary to edema in the mutant (F, arrowheads). Red blood cells have extravasated into the edematous area (F, arrow). All embryos were photographed at 30 hours postfertilization (hpf). PHENOTYPE:
|
|
The pfdgw1 mutation disrupts venous plexus and axial vessel formation. A-D: pfdgw1 was crossed into a fli1:EGFP transgenic line to allow visualization of endothelial cells. A: The caudal vein of wild-type embryos has a well-formed venous plexus (arrowheads). B: The caudal vein of pfdgw1 mutants has lost its characteristic reticular pattern, and endothelial cells are disorganized (arrowheads). C,D: Dorsal aorta (upper circle) and cardinal vein (lower circle) in a wild-type embryo (C) and a pfdgw1 mutant (D) demonstrating reduced axial vessel diameters in the mutant (D, circles). Embryos were photographed at 30 hours postfertilization (hpf; C,D) and 35 hpf (A,B). |
|
The outer layer of the notochord sheath is disrupted in pfdgw1 mutants. A-F: Transmission electron micrographs of truncal cross-sections from embryos at 30 hours postfertilization (hpf). A: Notochord sheath of a wild-type embryo (between arrows). The area in the white square is shown at higher magnification in panel C. B: Notochord sheath of a pfdgw1 mutant (between arrows). The area in the white square is shown at higher magnification in panel D. C: Notochord sheath of a wild-type embryo with inner (i), medial (m), and outer (o) layers. D: Notochord sheath of a pfdgw1 mutant where inner (i) and medial (m) layers are normal, but the outer (o) layer is reduced in size. E,F: Notochord sheaths of wild-type embryos treated with 10 μM neocuproine (E) or 10 mM &beta-aminopropionitrile (F). Not, notochord. PHENOTYPE:
|
|
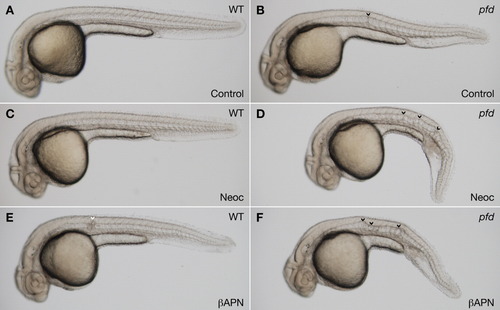
pfdgw1 mutants are sensitized to pharmacologic inhibition of lysyl oxidase. A-F: Clutches from pfdgw1/+ intercrosses were incubated in vehicle (A,B), the copper chelator neocuproine (2 μM; C,D), or the lysyl oxidase inhibitor β-aminopropionitrile (1 mM; E,F). Notochord is normal in wild-type embryos treated with vehicle or neocuproine (A,C) and shows a very mild herniation event in β-aminopropionitrile (E, arrowhead). Notochords of pfdgw1 mutants in neocuproine and β-aminopropionitrile (D,F, arrowheads) are substantially more distorted than mutants incubated in vehicle (B, arrowhead). Embryos were incubated in PTU to inhibit melanin pigmentation and photographed at 30 hours postfertilization (hpf). PHENOTYPE:
|
|
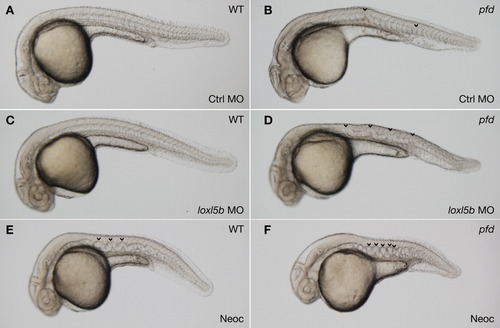
pfdgw1 mutants are sensitized to notochord distortion after partial knockdown of loxl5b. A-D: Embryos from pfdgw1/+ intercrosses were injected with 5 ng of control MO (A,B) or 5 ng loxl5b MO (C,D) and examined at 30 hours postfertilization (hpf). Neither morpholino induced notochord distortion in wild-type embryos (A,C). However, pfdgw1 mutants injected with lysyl oxidase morpholino developed striking notochord distortion (D, arrowheads) compared with mutants injected with control morpholino (B, arrowheads). E: Wild-type embryo incubated in high-dose (6 μM) neocuproine, demonstrating classic sine-wave appearance of lysyl oxidase inhibition at 30 hpf. F: pfdgw1 mutant at 30 hpf with irregular notochord distortion after incubation in 6 μM neocuproine. Embryos were treated with PTU to inhibit melanin pigmentation. PHENOTYPE:
|
|
The pfdgw1 mutation disrupts the zebrafish fbn2 gene. A: The pfdgw1 lesion was meiotically mapped to a telomeric region bounded by markers zC124A3 and z33723 on chromosome 22. The number of recombinants is noted for each marker; a single recombinant was identified at z33723. Marker, gene, and fibrillin expressed sequence tag (EST) locations relative to bacterial artificial chromosomes (BACs) and scaffolds (scfld) are illustrated on the physical map, which is based on Zv6. B: Sequencing traces from the fibrillin on chromosome 22 revealed a nonsense mutation (arrow) in pfdgw1 mutants that abrogates an AvaII restriction enzyme site. C: Genotyping of pfdgw1 fish by restriction digest of a polymerase chain reaction (PCR) product encompassing the mutated sequence. PCR product from the wild-type allele (+) is cleaved to generate fragments of 143 bp and 43 bp; product from the mutant allele (-) is not cleaved. D: Structure of zebrafish fibrillin-2 illustrating the conserved modular domains of this 2868 amino acid protein. The glycine-rich domain and location of the RGD motifs (asterisks) are characteristic of fibrillin-2 but not fibrillin-1 or fibrillin-3 orthologues. The pfdgw1 mutation is predicted to result in a truncated protein product (arrow). E: Structure of the three human fibrillins and zebrafish fibrillin-4. The percent amino acid identity with zebrafish fibrillin-2 and fibrillin-4 is indicated. The dashed line indicates sequence that is presumed to exist but has not been determined. F: Phylogenetic tree of fibrillin DNA coding sequences from various vertebrate species. Latent transforming growth factor-beta binding protein 1 (LTBP1) is used as the outgroup. Species are identified using standard two-letter abbreviations with zebrafish genes in bold. The arrow indicates Xenopus laevis fibrillin, a predicted fibrillin-2 orthologue. The scale bar reflects expected substitutions per site, and partial sequences begin with “p.” EXPRESSION / LABELING:
|
|
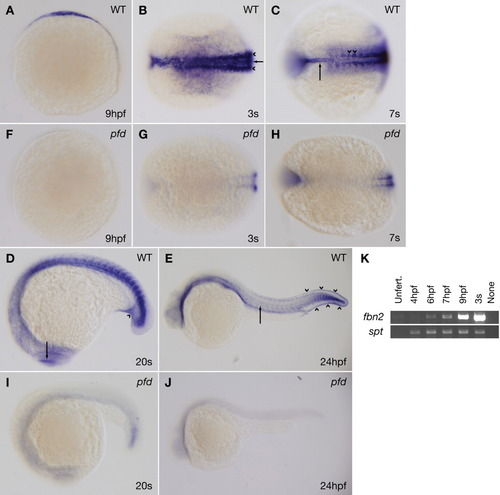
fbn2 expression is consistent with the pfdgw1 phenotype and dramatically reduced in pfdgw1 mutants. A-J: Clutches from pfdgw1/+ intercrosses were subjected to whole-mount in situ hybridization at the indicated developmental stages using probes to fbn2. A: Lateral view of a wild-type embryo at 9 hours postfertilization (hpf). B: Dorsal view of a wild-type embryo at the three-somite stage demonstrating fbn2 expression in the notochord (arrow) and paraxial mesoderm (arrowheads). C: Dorsal view of a wild-type embryo at the seven-somite stage demonstrating fbn2 expression in the notochord (arrow) and somites, with foci of increased staining near notochord-somite boundaries (arrowheads). D: Lateral view of a wild-type embryo at the 20-somite stage with fbn2 expression in the region of the developing caudal vein (arrowhead) and eye (arrow). E: Lateral view of a wild-type embryo at 24 hpf with hypochord (arrow) and prominent fin fold expression (arrowheads). F-J: fbn2 expression is dramatically reduced in pfdgw1 mutants at all stages analyzed. K: Reverse transcriptase-polymerase chain reaction (RT-PCR) for fibrillin-2 (fbn2) or spadetail (spt) using RNA from embryos at the indicated developmental stages. Unfert, unfertilized. PHENOTYPE:
|
|
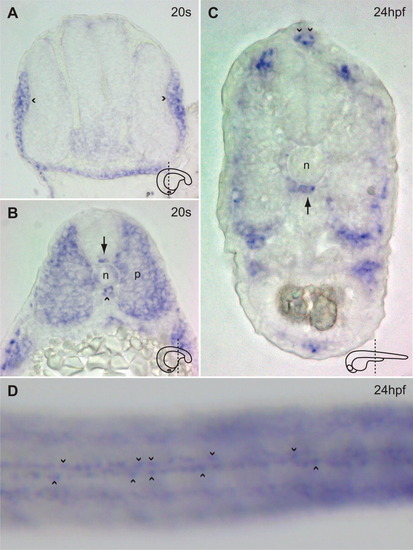
fbn2 expression in frozen sections (A-C), and at high-power magnification in a whole-mount specimen (D). A: Cross-section through the head of a wild-type embryo at the 20-somite stage demonstrating fbn2 expression in the lens placodes (arrowheads). B: Cross-section through the trunk of a wild-type embryo at the 20-somite stage demonstrating fbn2 expression in the notochord (n), floor plate (arrow), hypochord (arrowhead), and paraxial mesoderm (p). C: Cross-section through the trunk of a wild-type embryo at 24 hours postfertilization (hpf) demonstrating fbn2 expression in the hypochord (arrow) and fin fold (arrowheads). The plane of section is schematized for A-C. D: Dorsal view of a wild-type embryo at 24 hpf demonstrating fbn2 expression in two parallel lines of fin fold cells. EXPRESSION / LABELING:
|
|
pfdgw1 heterozygote embryos exhibit an intermediate level of fbn2 expression. A-C: Clutches from pfdgw1/+ intercrosses were subjected to whole-mount in situ hybridization at the 20-somite stage using probes to fbn2, photographed, and then genotyped. fbn2 expression is robust in wild-type embryos (A), dramatically reduced in mutant embryos (C), and intermediate in heterozygote embryos (B). EXPRESSION / LABELING:
PHENOTYPE:
|
|
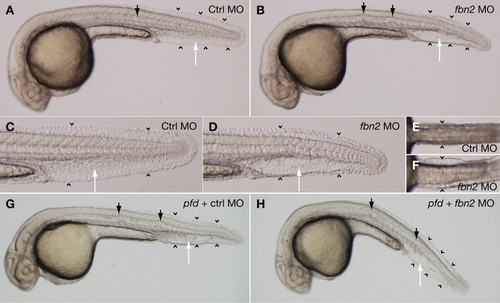
Morpholino knockdown of fbn2 recapitulates the pfdgw1 phenotype. A: Wild-type embryos injected with 2.4 ng of control morpholino exhibit normal notochord (black arrow), caudal vein (white arrow), and fin fold formation (arrowheads). B: Embryos injected with 2.4 ng of a start morpholino targeting fbn2 develop notochord kinks (black arrows), a cavernous caudal vein with loss of the usual reticular venous plexus (white arrow), and fin fold attenuation (arrowheads). C,D: The caudal vein and fin fold abnormalities in fbn2 morphants are better appreciated at higher magnification (D vs. C). E,F: Ventral views demonstrating truncal edema with skin distention in wild-type fish injected with fbn2 morpholino (F, arrowheads) but not control morpholino (E, arrowheads). G,H: pfdgw1 mutants injected with control morpholino (G) or fbn2 morpholino (H) are indistinguishable. All embryos were treated with PTU to inhibit melanin pigmentation and photographed at 30 hours postfertilization (hpf). Embryos in G and H were photographed and then genotyped. PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|