- Title
-
The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development
- Authors
- Insinna, C., Pathak, N., Perkins, B., Drummond, I., and Besharse, J.C.
- Source
- Full text @ Dev. Biol.
|
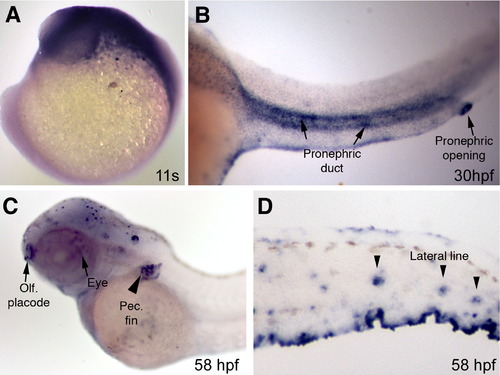
Analysis of Kif17 expression in whole embryos by in situ hybridization. (A) At the 11 somite stage, kif17 is expressed broadly in the head region. (B) At 30 hpf, Kif17 is observed in the pronephros and strongly in the pronephric opening (arrow). (C) At 58 hpf, expression is observed in the eye (arrow), olfactory placode (arrow), the forming pectoral fin (arrowhead), and panel D in the lateral line organs. EXPRESSION / LABELING:
|
|
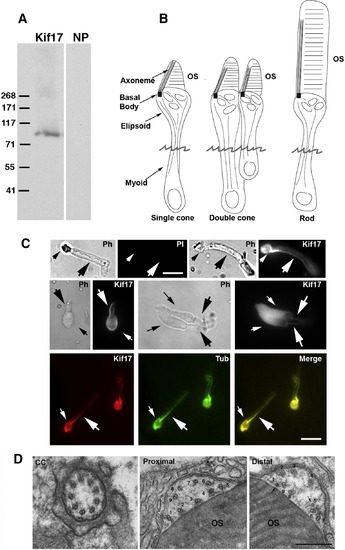
Immunocytochemical (IC) localization of Kif17 in photoreceptors. (A) Immunoblot of 3-day zebrafish embryo lysate. The Kif17 band corresponds to the predicted size based on the zebrafish cDNA sequence; NP, no primary antibody. (B) Three morphological types of zebrafish photoreceptors; isolated photoreceptors break off at the position of the jagged line. (C) IC of Kif17 in isolated adult zebrafish photoreceptors. Ph, Upper left is a phase (PH), and no primary antibody control image (PI) of a rod. Upper right is a phase and a Kif17 stained rod. Middle panel is a phase and Kif17 stained single cone (left), and double cone (right). Lower panel shows co-localization of Kif17 (left) with acetylated α-tubulin (middle) along the axoneme of a rod. Small arrows point to the ellipsoid region and the large arrows point to axoneme in the OS. Bar = 10 μm. (D) EM images at the level of the connecting cilium (CC), the proximal OS, and more distal OS of a developing cone from a 3-day zebrafish larva. Note that both the proximal and distal OS images are within the most proximal one-third of the OS. Doublets adjacent to the discs are numbered 1–9; the more distal image shows two singlets (1 and 5) and 7 doublets adjacent to the OS discs. Bar = 0.33 μm. EXPRESSION / LABELING:
|
|
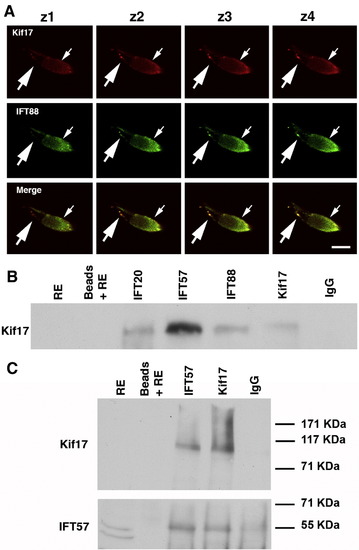
Association of Kif17 with IFT complex proteins. (A) Consecutive confocal Z-slices of a cone double labeled with Kif17 and IFT88 antibodies. The small arrow indicates the position of the ellipsoid and the large arrow points to punctate staining along the axoneme. Bar = 10 μm. (B) Co-immunoprecipitation of Kif17 with antibodies to IFT20, IFT57, IFT88, and Kif17. IP antibodies are shown at the top and western blot antibodies on the left. Kif17 was not detected in the RE, which was at 1/5 of the volume used for IP; Kif17 was readily detected when the extract load was higher (see Fig. 1A). Beads plus RE without antibody and a mixture of IgG1 and IgG2 were used as controls for non-specific binding. In separate gels, IFT antibodies were shown to co-IP using anti-IFT antibodies in zebrafish as previously shown for mouse retina (Baker et al., 2003). (C) IP experiment as in panel B in which the blot was cut into two pieces; the upper portion was stained for Kif17 and lower portion for IFT57. Note that as reported in mice (Pazour et al., 2002), two bands are detected with the IFT57 antibody but only the upper band is present in the co-IPs. EXPRESSION / LABELING:
|
|
Disruption of Kif17 splicing by an antisense morpholino oligonucleotide. (A) Mis-splicing of the Kif17 mRNA caused by blocking the exon 2 donor sequence. RT-PCR primers in exons 1 and 3 (small arrows) generate a smaller product in morphant embryos. (B) The morphant RT-PCR product sequence reveals an out of frame fusion to a cryptic splice donor within exon 2 resulting in a mRNA predicted to encode a Kif17 protein truncated at amino acid 102. (C) Morphant (mph) and control (invert sense) injected 3-day zebrafish larvae. (D) Western blots of 3-day embryos showing the reduction of Kif17 in morphants; anti-γ-tubulin was used as a loading control. EXPRESSION / LABELING:
|
|
Disrupted photoreceptor OS formation in Kif17 morphants. (A) WT eye of a 3-day-old embryo. The lens and retinal lamination are well developed. OSs (small arrows, center inset) have developed throughout the retina. G, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer (photoreceptor nuclei). Bar = 10 μm. (B) EM view of junction between photoreceptors and retinal pigment epithelium (RPE) in WT showing mitochondria rich inner segments (IS) and the lamellar OSs; N, photoreceptor nucleus. Bar = 2.5 μm. (C) Morphant eye of a 3-day-old embryo shows normal retinal lamination but lack of OS (small arrow, center inset). Bar = 10 μm. (D) EM view of junction between photoreceptors and RPE in a morphant; IS layer is visible between nuclei (ONL) and RPE, but OS are not present. Bar = 5 μm. (E) EM view of junction between photoreceptors and RPE of a morphant with short, under-developed OS (arrows). Bar = 6.6 μm. (F) High power EM view of a photoreceptor showing the basal body (BB) at the base of the cilium; arrow indicates disorganized OS discs. M, mitochondrion. OS (upper right) is from an adjacent photoreceptor. Bar = 0.5 μm. PHENOTYPE:
|
|
Correlation between photoreceptor OS loss and extent of Kif17 knock-down in morphants. (A–C) Kif17 distribution in eyes of 3-day-old control (A) and morphant larvae (B, C). (D–F) IC images (A–C) merged with bright-field images of the same sections. Abbreviations: GCL, ganglion cell layer; INL, inner nuclear layer; and ONL, outer nuclear layer. Scale bars in panels D–F = 40 μm. Note that in the photoreceptor layer of the early developing central retina (asterisks) of morphants Kif17 immunoreactivity is almost completely lacking but in the late developing periphery (indicated by arrows), Kif17 protein is present. (G–I) EM images from the central region of a control retina (G), and of a morphant retina (H). (I) is from the peripheral region of a morphant retina. Note that OS form throughout the retina of controls and are virtually absent from the central retina of morphants where Kif17 protein knock-down is most complete. OS are seen in the periphery of some but not all morphants. EM magnification bars: 2.8 μm (G), 4.0 μm (H), and 6.8 μm (I). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Mis-localization of the photopigment apoprotein in cones and to a lesser extent in rods of morphants. (A) (Upper panel) Double label of rhodopsin (green) and CNG1 (red) in rods of 3-day-old control (WT) and morphants. Topro (blue) was used in the control (WT) to show the nuclear layers. Note that Rho and CNG1 co-localize in the OS in control (WT) and are undetectable in the outer plexiform layer (arrows). (Lower panel) In contrast to control (WT), both Rho and CNG1 are seen in the outer plexiform (synaptic) layer of morphants (arrows). Bar = 20.55 μm. (B) Cone opsin (green) is very extensively mis-localized to the perinuclear region and outer plexiform layer (synaptic layer) in morphant (mph) cones compared to control (see arrows). Sections are double labeled with Topro (blue) to show the nuclear layers. Bar = 12.5 μm. EXPRESSION / LABELING:
PHENOTYPE:
|

Unillustrated author statements EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 316(1), Insinna, C., Pathak, N., Perkins, B., Drummond, I., and Besharse, J.C., The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development, 160-170, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.