- Title
-
RFX2 is essential in the ciliated organ of asymmetry and an RFX2 transgene identifies a population of ciliated cells sufficient for fluid flow
- Authors
- Bisgrove, B.W., Makova, S., Yost, H.J., and Brueckner, M.
- Source
- Full text @ Dev. Biol.
|
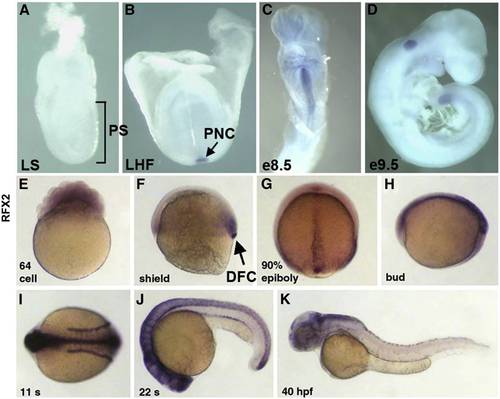
Mouse and zebrafish homologues of rfx2 are expressed in ciliated organs of asymmetry and other ciliated tissues. In situ localization of mouse Rfx2 transcripts indicated no expression in late streak (LS) or earlier stage embryos (A). Expression was first detected in the posterior notochord (PNC) at late headfold stage (B), persists in the midline through e8.5 (C) and was downregulated in all but the posterior tail by e9.5 (D). In situ localization of zebrafish rfx2 indicated that transcripts are present maternally (E). At shield stage zygotic expression was limited to mesendoderm at the margin and dorsal forerunner cells (DFC) (F). During late epibiloy (G) and bud stages (H) rfx2 transcripts are restricted to the embryonic midline. During somite stages, expression was restricted to the neural tube and pronephric ducts (I,J) and becomes down-regulated everywhere except regions of the dorsal brain by 40 h post-fertilization (K). |
|
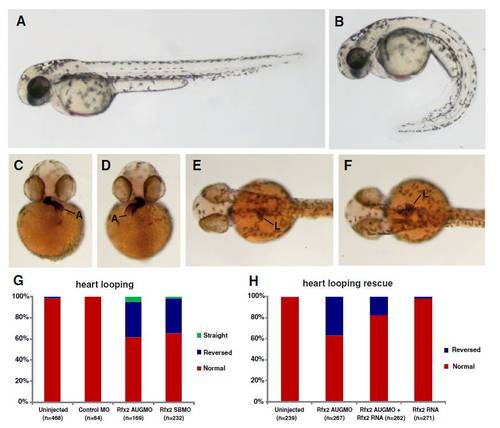
Knockdown of rfx2 gene function results in altered body shape, and LR patterning defects in heart and gut. Rfx2 morphants (B) exhibited a strongly ventrally curved trunk and tail compared to un-injected wild-type embryos (A). The direction of heart and gut looping were often reversed in rfx2 morphants (C?H). In a wild-type embryo, the atrium of the heart loops toward the left side of the embryo (C) and the liver and intestinal bulb are located on the left side of the embryo (E) while in rfx2 morphants the position of these organs was often reversed (D, F). B, D, F - rfx2 AUGMO. Histogram depicting the percentage of heart and gut reversals in wild-type and rfx2 morphant embryos (G). The incidence of gut reversals in rfx2 AUGMO embryos was partially rescued by injection of rfx2 RNA. (H) Histogram depicting the percentage of heart reversals in wild-type and rfx2 AUGMO embryos injected with rfx2 RNA. PHENOTYPE:
|
|
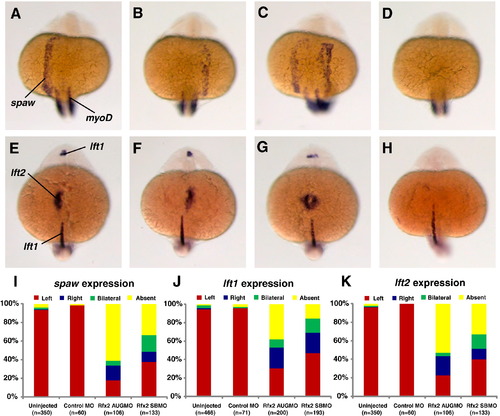
Rfx2 functions upstream of early asymmetric LPM genes. In 19?21 somite-stage rfx2 morphants spaw was expressed in the lateral plate mesoderm on the left side (A, typical of wt embryos) as well as on the right side (B), bilaterally (C), or was not expressed (D). At the 22?24 somite-stage, the two lefty genes are expressed asymmetrically, with lft1 predominantly in the left diencephalon and lft2 expressed in the left heart field. Individual rfx2 morphants displayed this normal left-sided expression (E), or aberrant right-sided expression in both the diencephalon and heart field (F), bilateral expression in both (G), or an absence of expression (H). Midline expression of lft1 was normal in morphants. myod1 expression in somites was used to stage embryos. Embryos shown are rfx2 AUGMO; rfx2 SBMO are similar. Histograms depicting the percentages of spaw (I), lft1 (J) and lft2 (K) expression patterns in wild-type and rfx2 morphant embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
|
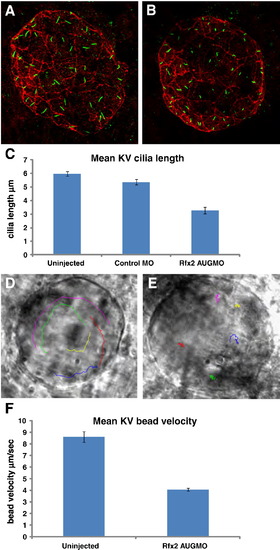
Rfx2 knockdown disrupts ciliogenesis in KV and KV fluid flow. Cilia within Kupffer′s vesicle of 8?9 somite-stage embryos injected with control morpholino averaged 5.34 μm in length (A) while those in embryos injected with rfx2 AUGMO were significantly shorter, averaging 3.28 μm in length (B,). (C) Histogram of mean cilia length of KV cilia in un-injected, control MO and rfx2 AUGMO embryos (9 embryos each, analysis by one-way ANOVA, error bars are standard error) Fluorescent beads injected into KV of 8 somite-stage control embryos move in a counterclockwise direction (D; Supplemental Video 1A). In rfx2 AUGMO embryos there is no net directional flow and injected beads follow a random trajectory, often reversing direction (E; Supplemental Video 1B). The embryos in panels D, E are oriented with the embryonic notochord in the lower right-hand corner of the image. (F) Histogram of mean bead velocities in un-injected control embryos and rfx2 AUGMO embryos (6 control embryos, 7 rfx2AUGMO; 5 beads tracked in each, analysis by Student′s two tailed t-test, error bars are standard error). EXPRESSION / LABELING:
PHENOTYPE:
|
|
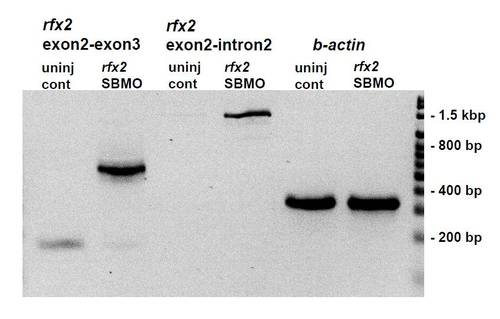
RT-PCR of cDNA prepared from total RNA extracted from bud stage un-injected zebrafish embryos and rfx2 SBMO injected embryos. Injection of 8 ng of rfx2 SBMO causes loss of the 170 bp wild-type rfx2 product (compare lanes 1 and 2) and retention of the intron separating exon 2 (the first coding exon) and exon 3. Much of the intron-containing product undergoes splicing at a cryptic splice donor site 369 bp into the intron (lane 2) while some remains unspliced (lane 4) and reads through this site to (and beyond) the Intron 2R primer located at 1270 bp into the intron. PCR products retaining the entire second intron are recovered using extended elongation times with the Exon2L/Exon3R primer pair (not shown). Lanes 5, 6 β-actin loading controls. Primers: Exon 2 L: (52 ? GTCAGAAGGGGGCTCAGAGA-32); Intron 2R: (52- TCTAGCAGTGTGGCCGGTAT); Exon 3R; (52- TGTTGCACTCTAGGCACTGG-32). |
Reprinted from Developmental Biology, 363(1), Bisgrove, B.W., Makova, S., Yost, H.J., and Brueckner, M., RFX2 is essential in the ciliated organ of asymmetry and an RFX2 transgene identifies a population of ciliated cells sufficient for fluid flow, 166-178, Copyright (2012) with permission from Elsevier. Full text @ Dev. Biol.