- Title
-
Control of convergent yolk syncytial layer nuclear movement in zebrafish
- Authors
- Carvalho, L., Stühmer, J., Bois, J.S., Kalaidzidis, Y., Lecaudey, V., Heisenberg, C.P.
- Source
- Full text @ Development
|
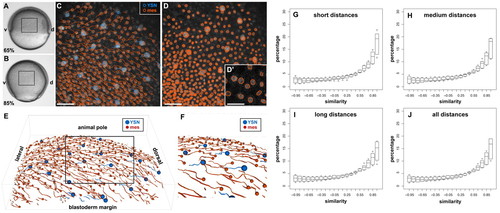
Convergence movements are highly coordinated between iYSN and mesendoderm. (A,B) Bright-field images of an embryo at the beginning of gastrulation (6.5 hpf; A) and at mid-gastrulation (8.5 hpf; B). Animal pole is towards the top and dorsal is towards the right. Boxes delineate the imaged region in C-E. (C,E,F) Trajectories of iYSN and mesendoderm progenitors during gastrulation were obtained by imaging embryos by 2-photon excitation microscopy from 65% epiboly stage (6.5 hpf) until 85% epiboly stage (8.5 hpf), and analyzing these movies with a newly developed 3D tracking software. Dorsal is towards the right and circles indicate the endpoint of each track. (C) Z-projection showing iYSN (blue tracks) and mesendoderm (orange tracks) nuclear trajectories. (D) Z-projection depicting the efficacy of the image segmentation algorithm to detect nuclei in 3D. (D′) Magnification of a single slice of the image in D. The segment boundaries (in orange) are plane cuts of the ellipsoids detected by the image segmentation algorithm. Large ellipsoids represent iYSN, small ellipsoids represent cell nuclei. (E,F) 3D views of the trajectories of iYSN (blue tracks) and mesendoderm progenitors (orange tracks). (F) Magnification of boxed region in E. (G-J) Quantification of similarity values between iYSN and mesendoderm progenitors in wild-type embryos at mid-gastrulation stages (7-8 hpf). Histograms of the similarity values were generated separately for each embryo (n=6 embryos). Box plots show the distribution of the bin heights among the different embryos. (G) Similarity at short distances (0-40 μm). On average, 52% of the values are higher than 0.5. (H) Similarity at medium distances (40-80 μm). On average, 50% of the values are higher than 0.5. (I) Similarity at long distances (80-120 μm). On average, 48% of the values are higher than 0.5. (J) Similarity distribution considering all distances together. On average, 50% of the values are higher than 0.5. d, dorsal; v, ventral; mes, mesendoderm. Scale bars: 50 μm in C,D; 25 μm in D′. |
|
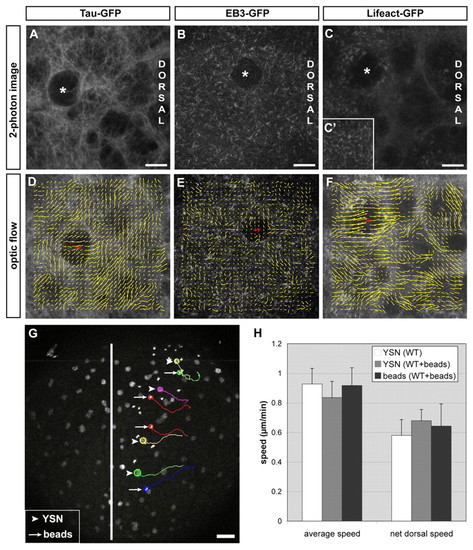
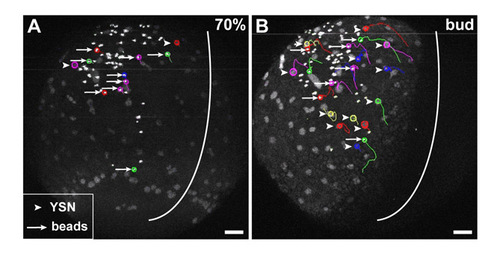
iYSN converge by cortical flow within the YSL. (A-C′) Microtubules labeled with Tau-GFP (A), microtubule plus-ends labeled with EB3-GFP (B) and actin labeled with Lifeact-GFP (C,C′) in the paraxial region of the iYSL at 90% epiboly (9 hpf; A,C) and bud stage (10 hpf; B). Asterisks mark iYSN. (C′) Dotty actin staining in more superficial layers of the YSL and filamentous actin in deeper layers (C). (D-F) Vector fields showing optical flow direction (yellow arrows) of Tau-GFP (D), EB3-GFP (E) and Lifeact-GFP (F). Red arrows indicate the movement direction of the iYSN. (G) Two-photon image of two-somite stage wild-type embryo with fluorescently labeled YSN and 0.5 μm diameter fluorescent microspheres injected into the YSL. Image is a z-projection. Several nuclear (arrowheads) and microsphere (bead) trajectories (arrows) obtained using Motion Tracking Software are shown. Circles indicate the endpoint of each track. The white line indicates the dorsal midline of the embryo. (H) Comparison of the speed of YSN and bead movements in control embryos (n=6 embryos) and embryos injected with beads (n=2 embryos). No significant differences were found in average and net dorsal speed of YSN and beads (P>0.05). Notably, injected beads appear to have slightly less persistent movements compared with adjacent iYSN (see Movie 4 in the supplementary material). This reduction in bead persistence is probably due to the smaller diameter of the beads (0.5 μm) compared with iYSN (6-20 μm), resulting in greater diffusive relative to advective motion for beads in comparison with iYSN. Dorsal is towards the right (A-F) and animal is towards the top (A-G). Scale bars: 10 μm in A-C; 50 μm in G. |
|
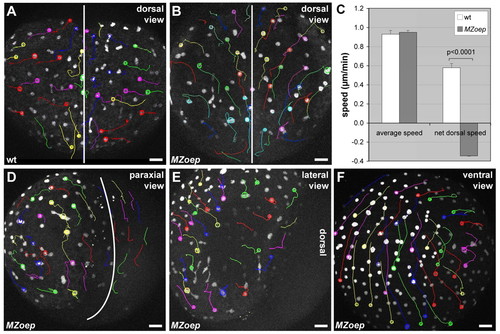
iYSN convergence movements are impaired in MZoep mutant embryos. (A,B,D-F) iYSN trajectories in wild-type (A) and MZoep (B,D-F) embryos at the two-somite stage (11 hpf) in dorsal (A,B), paraxial (D), lateral (E) and ventral (F) view. Images are z-projections. Some of the nuclear trajectories obtained using Motion Tracking Software are shown. Circles indicate the endpoint of each track. The white lines in A,B,D mark the dorsal midline of the embryo. Animal pole is towards the top. (A) In wild-type (wt) embryos, iYSN converge from lateral and paraxial regions towards the dorsal side of the embryo, and undergo longitudinal movements along the anteroposterior axis of the gastrula. (B) In MZoep, iYSN from lateral and paraxial regions fail to converge to the dorsal side, but still undergo longitudinal movements in an anterior direction. (C) Comparison of the speed of iYSN movements between wild-type (wt) and MZoep embryos. The average speed of iYSN is similar in wild-type and MZoep embryos [P=0.7412; n=6 (wt); n=2 (MZoep)]. By contrast, the net dorsal speed is significantly lower in MZoep embryos compared with wild type [P<0.0001; n=6 (wt); n=2 (MZoep)]. The negative MZoep value indicates that the net dorsal movement of the nuclei is away from the dorsal midline. Unpaired Student's t-tests were performed to test the differences between mean values. Error bars represent the standard error of the mean. (D-F) iYSN from paraxial, lateral and ventral regions in MZoep mutants show little convergence movements to the dorsal side. In F, the embryo is slightly tilted, and thus some lateral iYSN are observed on the right side of the image moving away from the dorsal region. |
|
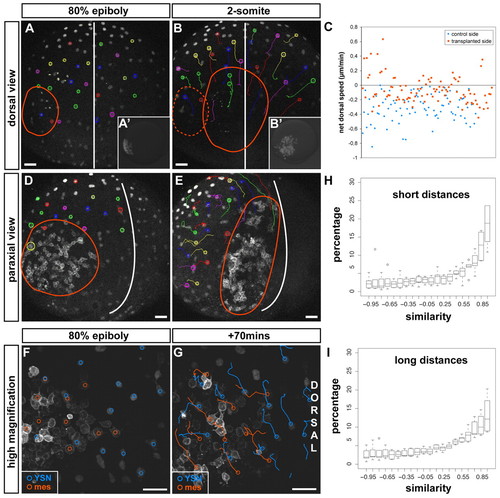
Mesendoderm directs iYSN convergence movements. (A,B,D,E) Trajectories of iYSN in MZoep embryos containing transplanted mesendoderm cells at 80% epiboly (8 hpf; A,D, startpoint of tracks) and at the two-somite stage (11 hpf; B,E, endpoint of tracks). The red line marks the position of the transplanted cells. (A,B) iYSN convergence movements at the side of MZoep embryos containing transplanted mesendoderm cells versus iYSN on the untransplanted side. Dorsal view. (A′,B′) Images of rhodamine dextran-labeled transplanted cells in the same MZoep embryo in A,B before (8 hpf; A′) and after (11 hpf; B′) the timelapse. (C) Quantification of iYSN net dorsal speed shows that iYSN convergence movements at the side containing transplanted mesendoderm cells is significantly increased compared with the convergence of iYSN at the untransplanted side (P<0.0001; n=197 iYSN from four embryos). Unpaired Student's t-tests were performed to test the differences between the mean values. (D,E) Paraxial view of a transplanted MZoep embryo shows that lateral iYSN moving behind the transplanted cells (labeled with membrane-bound GFP) undergo convergence movements similar to the transplanted cells. (F,G) Trajectories of iYSN (blue tracks) and transplanted cells labeled with membrane-bound GFP (orange tracks) in a transplanted MZoep embryo at 80% epiboly (8 hpf; F, startpoint of tracks) and 70 minutes later (G, endpoint of tracks). Images are z-projections. Dorsal is towards the right and animal is towards the top. iYSN underneath and in front of the transplanted cells move dorsally and posteriorly together with the cells. (H,I) Quantification of movement similarity between iYSN and transplanted cells. Histograms of the similarity values were generated separately for each experiment (n=6). Boxplots show the distribution of the bin heights among the experiments. Circles indicate outliers. (H) Similarity at short distances (0-40 μm). On average, 54% of the values are higher than 0.5. (I) Similarity at long distances (80-120 μm). On average, 48% of the values are higher than 0.5. Considering all distances together, 50% of the values are higher than 0.5 (not shown). Scale bars: 50 μm. |
|
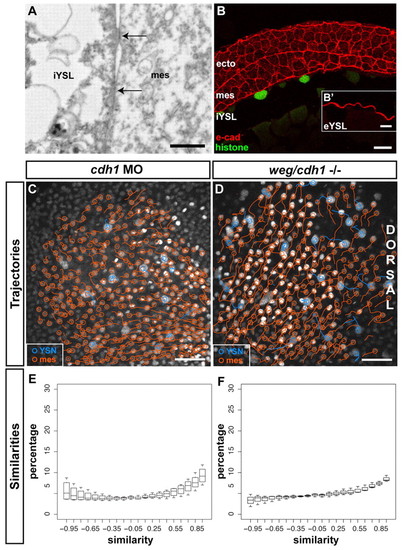
E-cadherin is required for convergence movement coordination between iYSN and mesendoderm. (A) Sagittal section obtained by transmission electron microscopy of a wild-type embryo at 80% epiboly stage (8 hpf) showing a mesendoderm progenitor in close contact with the iYSL plasma membrane. Note the presence of electron-dense regions (arrows) of tight contact between the mesendoderm progenitor and the iYSL plasma membrane. Animal pole is towards the right. (B) E-cadherin antibody staining of transversal (B) and sagittal (B′) sections of wild-type gastrulating embryos. E-cadherin is localized at ectoderm, mesendoderm and YSL plasma membranes. (B′) Magnification of the eYSL region. (C,D) Trajectories of iYSN (blue tracks) and mesendoderm progenitors (orange tracks) in e-cadherin morphant (C) and weg/e-cadherin mutant (D) embryos at mid-gastrulation stages (7-8 hpf). Images are z-projections. Dorsal is towards the right. (E,F) Quantification of the similarity between iYSN and mesendoderm convergence movements in e-cadherin morphants (E) and weg/e-cadherin mutants (F) at mid-gastrulation stages (7-8 hpf). Histograms of the similarity values were generated separately for each embryo. Boxplots show the distribution of the bin heights among the embryos. (E) In e-cadherin morphants, 35% of the similarity values are higher than 0.5 (n=4 embryos). (F) In weg/e-cadherin mutants, 35% of the similarity values are higher than 0.5 (n=3 embryos). e-cad, e-cadherin; ecto, ectoderm progenitor; mes, mesendoderm progenitor. Scale bars: 2 μm in A; 20 μm in B; 10 μm in B′; 50 μm in C,D. |
|
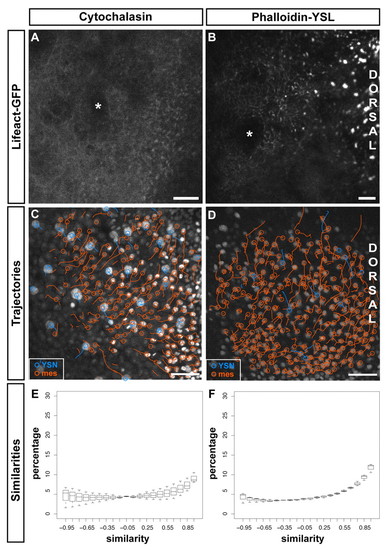
Filamentous actin within the YSL is required for convergence movement coordination between iYSN and mesendoderm. (A,B). Actin labeled with Lifeact-GFP in embryos incubated in cytochalasin (A) and injected with Phalloidin into the YSL (B) at 90% epiboly (9 hpf; A) and bud stage (10 hpf; B). Asterisks mark iYSN. (C,D) Trajectories of iYSN (blue tracks) and mesendoderm progenitors (orange tracks) in cytochalasin-treated (C) and phalloidin YSL-injected (D) embryos at mid-gastrulation stages (7-8 hpf). Images are z-projections. Dorsal is towards the right. (E,F) Quantification of the similarity between iYSN and mesendoderm convergence movements in cytochalasin-treated (E) and phalloidin YSL-injected (F) embryos at mid-gastrulation stages (7-8 hpf). Histograms of the similarity values were generated separately for each embryo. Box plots show the distribution of the bin heights among the embryos. (E) In cytochalasin-treated embryos, 34% of the similarity values are higher than 0.5 (n=3 embryos). (F) In phalloidin YSL-injected embryos, 41% of the similarity values are higher than 0.5 (n=3 embryos). mes, mesendoderm progenitor. Scale bars: 2 μm in A; 20 μm in B; 50 μm in C,D. |
|
Cortical flow within the YSL is altered in MZoep mutant embryos. (A,B) Trajectories of iYSN (arrowheads) and 0.5 μm diameter fluorescent microspheres (beads; arrows) in the YSL in 70% epiboly (7 hpf; A) and bud stage (10 hpf; B) MZoep mutant embryo. Images are z-projections. Some nuclear and bead trajectories obtained using Motion Tracking Software are shown. Circles indicate the endpoint of each track. White line indicate the dorsal midline of the embryo. Animal is towards the top. Scale bars: 50 μm. |
|
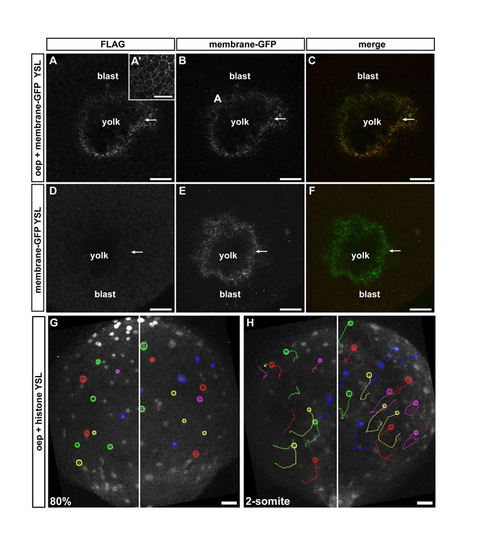
Overexpression of Oep within the YSL does not rescue defective iYSN convergence movements in MZoep mutant embryos. (A-C) Oep-FLAG antibody staining in MZoep embryos overexpressing oep in the YSL during gastrulation. The YSL of MZoep embryos were injected with 400 pg oep-FLAG and 100 pg membrane-bound gap43-GFP. Confocal transversal sections showing expression of Oep-FLAG within the YSL (A), which colocalize with membrane-bound GFP (B,C; arrows). (A′) Oep-FLAG antibody staining in an MZoep embryo injected with 200 pg oep-FLAG at the one-cell stage, showing expression of Oep on the cell membranes of blastoderm cells. (D,E) MZoep embryo injected with 100 pg membrane bound-GFP into the YSL. Confocal transversal section showing no detection of the FLAG tag (D), whereas membrane-bound GFP is strongly expressed in the YSL (E,F, arrows). (A,D) Flag antibody staining. (B,E) Membrane-bound GFP. (C,F) Merged image of FLAG and GFP channels. (G,H) iYSN trajectories in MZoep mutant injected with oep-FLAG into the YSL at 80% epiboly (8 hpf; G, startpoint of tracks) and two-somite stage (11 hpf; H, endpoint of tracks). Images are z-projections. Some nuclear trajectories obtained using Motion Tracking Software are shown. Circles indicate the endpoint of each track. White line marks the dorsal midline of the embryo. Animal is towards the top. Blast, blastoderm. Scale bars 40 μm in A-F; 50 μm in G,H. |
|
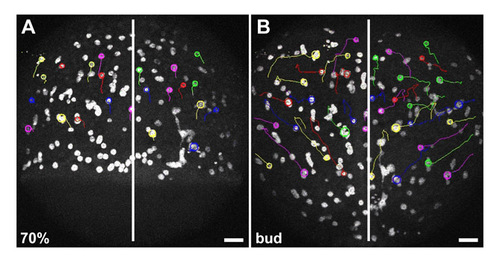
Endoderm progenitors are not required for iYSN convergence movements. (A,B) iYSN trajectories in cas mutant embryos at 70% epiboly (7 hpf; A, startpoint of tracks) and bud stage (10 hpf; B, endpoint of tracks). iYSN undergo normal convergence movements. Images are z-projections. Some nuclear trajectories obtained using Motion Tracking Software are shown. Circles indicate the endpoint of each track. Dorsal views. White line marks the dorsal midline of the embryo. Animal is towards the top. Scale bars: 50 μm. |