|
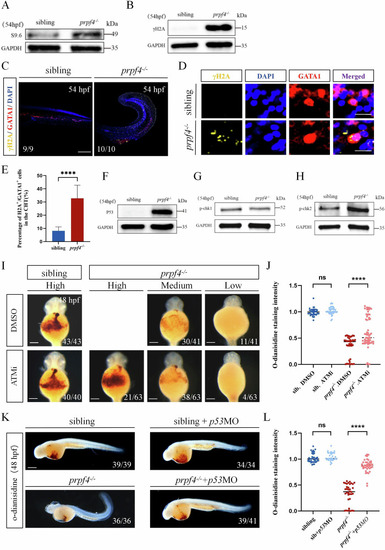
Activating of the DDR-ATM/Chk2-p53 signaling pathway disrupts erythropoiesis in prpf4 mutants. A, B Western blot analysis for RNA:DNA hybrid accumulation and DNA damage in 54 hpf embryos. A S9.6 antibody detected higher RNA:DNA hybrid levels in prpf4−/− embryos than in siblings. B γH2AX levels are significantly increased in prpf4−/− embryos compared with siblings. C–E Analysis of DNA damage in GATA1+ erythrocytes in 54 hpf embryos. C Confocal images show γH2AX staining in the CHT region of siblings and prpf4−/− embryos. Scale bar: 200 µm. D High-magnification images of erythrocytes show the colocalization of γH2AX and GATA1. Scale bar: 50 µm. E Quantification of the percentage of γH2AX-positive cells among GATA1+ cells in the CHT region. F-H Western blot analysis of the activation of P53 and checkpoint kinase signaling in 54 hpf embryos. F Increased P53 protein levels are observed in prpf4−/− embryos compared with siblings. G p-Chk1 protein levels show no significant changes between prpf4−/− embryos and siblings. H p-Chk2 protein levels are increased in prpf4−/− embryos compared with siblings. I, J Effect of the specific kinase inhibitor ATMi (KU60019) on erythrocyte hemoglobin levels in prpf4−/− embryos at 48 hpf. I O-dianisidine staining intensity in DMSO-treated siblings, prpf4−/− embryos, and ATMi-treated prpf4−/− embryos. Scale bar: 100 µm. J Quantitative analysis of o-dianisidine staining intensity. ATMi-treated prpf4−/− embryos show significantly higher staining intensity compared to DMSO-treated prpf4−/− embryos. K, L Effect of p53 knockdown on erythrocyte hemoglobin levels in prpf4−/− embryos at 48 hpf. K Knockdown of p53 in prpf4−/− embryos significantly increased hemoglobin levels, as indicated by stronger o-dianisidine staining. Scale bar: 200 µm. L Quantitative analysis of o-dianisidine staining intensity.
|