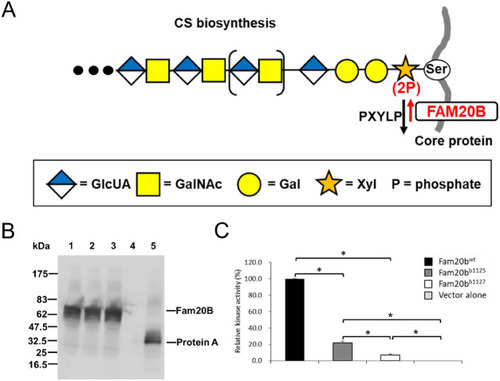
Mutant Fam20b proteins have severely hypomorphic kinase activities. (A) Model illustrating that Fam20b transiently phosphorylates xylose, the first sugar added to a serine residue of the core protein during synthesis of a chondroitin sulfate PG (Koike et al., 2009). 2-Phosphoxylose phosphatase (PXYLP) removes the phosphate, thus promoting efficient glycosaminoglycan side chain outgrowth (Koike et al., 2014). (B) Culture medium from COS-1 cells transfected with secreted forms of zebrafish Fam20b or vector alone was incubated with IgG-Sepharose, and proteins purified from the medium were subjected to SDS-PAGE. Expression of each protein A-tagged protein was examined with anti-mouse IgG antibody. Lane 1: Fam20bwt; lane 2: Fam20bb1125; lane 3: Fam20bb1127; lane 4: protein marker; lane 5: vector alone. (C) After normalization from immunoblots in B, evaluation of kinase activity demonstrated that the mutant kinases Fam20bb1125 and Fam20bb1127 both have a significant decrease in activity compared with Fam20bwt, with Fam20bb1127 displaying significantly less activity than Fam20bb1125. Both mutant kinases, however, showed more activity than the negative, empty-vector control. Data are mean+s.e.m. (n=3, Tukey's multiple comparison test, *P<0.05). CS, chondroitin sulfate; Gal, galactose; GalNAc, N-acetylgalactosamine; GlcUA, glucuronic acid; P, phosphate; Ser, serine; Xyl, xylose.
|