- Title
-
Male germ cell-associated kinase (MAK) is required for axoneme formation during ciliogenesis in zebrafish photoreceptors
- Authors
- Chiang, H.J., Nishiwaki, Y., Chiang, W.C., Masai, I.
- Source
- Full text @ Dis. Model. Mech.
|
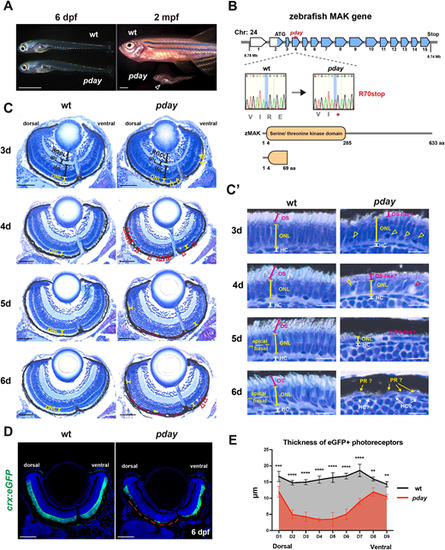
The zebrafish mak mutant pday shows photoreceptor degeneration. (A) pday mutants at 6 days post fertilization (dpf) and 2 months post fertilization (mpf). At 6 dpf, pday mutants showed no morphological differences from wild-type (wt) siblings. At 2 mpf, pday mutants had smaller bodies with less-developed fins, scoliosis and cardiac edema (open arrowhead). (B) The zebrafish mak gene consists of 15 exons and encodes 633 amino acids (aa) with a serine/threonine kinase domain. In pday mutants, a nonsense mutation (R70stop) results in a truncated protein lacking most of the kinase domain. (C) Wild-type and pday mutant retinas. The outer nuclear layer (ONL) in pday mutants showed dense nuclear granules (yellow arrowheads), bubble-like structures (red arrowheads) and reduced ONL thickness (yellow lines). In pday mutants, the dorso-central ONL progressively decreased in thickness after 4 dpf, became extremely flat at 5 dpf and disappeared at 6 dpf (red dotted lines). INL, inner nuclear layer; IPL, inner plexiform layer; RGCL, retinal ganglion cell layer. (C′) Higher magnification of the central ONL. The outer segment (OS) was drastically reduced in pday mutants (red lines). Dense nuclear granules (yellow arrowheads), bubble-like structures (red arrowheads) and the progressive reduction of ONL thickness (yellow lines) were observed in pday mutants. HC, horizontal cells; PR, photoreceptors. (D) Wild-type and pday mutant retinas carrying the transgenic line Tg[crx:eGFP]. Nuclei were counterstained with Hoechst 33342 (blue). crx:eGFP (green) expression was more severely decreased in the dorsal retina (red dotted line) than in the ventral retina of pday mutants. (E) Thickness of eGFP-positive photoreceptors along the dorso-ventral axis of the retina. The thickness was more severely decreased in the dorsal retina than in the ventral retina of pday mutants. Color bars and lines indicate mean±s.d. Statistical difference was evaluated with two-way ANOVA and Sidak's multiple comparison tests; n=3 for each point. **P<0.01; ***P<0.001; ****P<0.0001. Sample sizes are shown in Table S3. Scale bars: 1 mm (A); 50 µm (C); 10 µm (C′); 40 µm (D). |
|
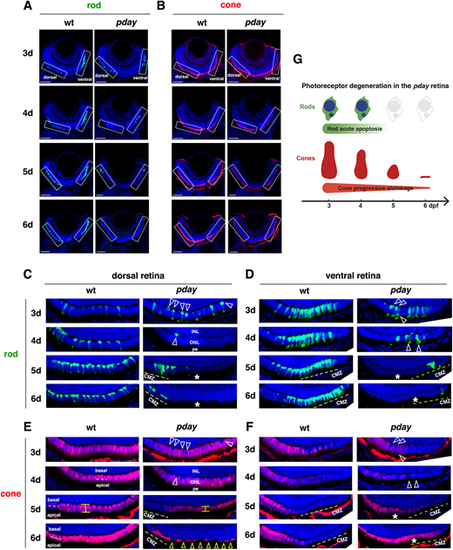
Rods undergo apoptotic-like degeneration in pday mutants, whereas cones progressively shrink. (A,B) Images of wild-type and pday mutant retinas carrying the transgenes Tg[rho:NLS-eGFP] and Tg[gnat2:NLS-tdTomato], which visualize rods (green, A) and cones (red, B), respectively. Nuclei were counterstained with Hoechst 33342 (blue). Rectangles indicate the dorsal and ventral ONLs, shown in C-F. (C) The dorsal ONL of wild-type and pday mutant retinas with Tg[rho:NLS-eGFP] (green). In pday mutants, rods had condensed, round nuclei (white arrowheads) at 3 dpf, and rods were drastically reduced in number at 4 dpf and disappeared at 5 and 6 dpf (asterisks), except in the peripheral region near the dorsal ciliary marginal zone (CMZ). pe, retinal pigment epithelium. (D) The ventral ONL of wild-type and pday mutant retinas with Tg[rho:NLS-eGFP] (green). In pday mutants, rods had condensed, round nuclei (white arrowheads) at 3 and 4 dpf white dotted lines. In pday mutants, rods disappeared at 5 and 6 dpf (asterisks), except in the peripheral region near the ventral CMZ. (E) The dorsal ONL of wild-type and pday mutant retinas with Tg[gnat2:NLS-tdTomato] (red). In wild type, cones formed a monolayer at 3 dpf and two subnuclear layers after 4 dpf (white dotted lines). In pday mutants, tdTomato-negative nuclei were observed, the positions of which were identical to those of condensed rod nuclei (white arrowheads). From 4 to 5 dpf, cones shrunk progressively and became flattened (yellow line). At 6 dpf, cones were extremely flattened and disappeared, leaving gaps (yellow arrowheads), except in the peripheral region near the dorsal CMZ. (F) The ventral ONL of wild-type and pday mutant retinas with Tg[gnat2:NLS-tdTomato] (red). In wild type, cones differentiated at 3 dpf, but progressively disappeared toward the periphery of the ventral ONL, where rods densely differentiated. In pday mutants, tdTomato-negative round nuclei appeared at the place where pyknotic-like rod nuclei were observed (white arrowheads, D) at 3 dpf, and ONL thickness was progressively decreased after 4 dpf. Interestingly, after 5 dpf, the cone differentiating area was expanded toward the periphery of the ventral ONL (asterisks), where rods disappeared. (G) Cone and rod degeneration process in pday mutants. Rods undergo acute degeneration with apoptotic-like pyknotic nuclei during 3-4 dpf, whereas cones undergo progressive shrinkage of cell volume during 4-6 dpf and disappear by 6 dpf. Sample sizes are shown in Table S3. Scale bars: 40 µm (A,B). |
|
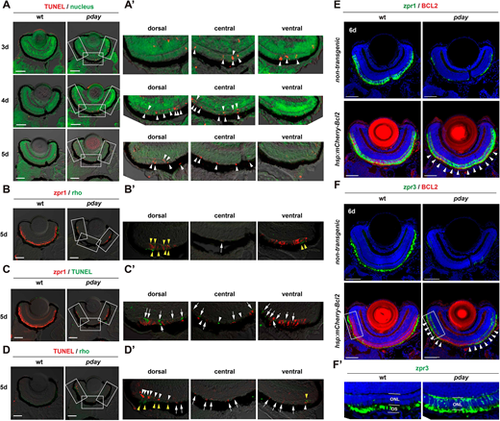
Rods and cones undergo apoptosis in pday mutants. (A) TUNEL (red) of wild-type and pday mutant retinas. Nuclei were counterstained with Sytox Green (green). (A′) Higher magnification of the dorsal, central and ventral ONL of pday mutant retinas, which are indicated by white rectangles in A. White arrowheads indicate TUNEL signals (red). (B) Double labeling of 5-dpf wild-type and pday mutant retinas with zpr1 (red) and anti-rhodopsin (green) antibodies. (B′) Higher magnification of the dorsal, central and ventral ONL of pday mutant retinas, which are indicated by white rectangles in B. Both rhodopsin (yellow arrowheads) and zpr1 signals were observed in the peripheral region of the dorsal and ventral ONL. In addition, zpr1-positive flattened cells were located in the central ONL (white arrow). (C) TUNEL (green) of 5-dpf wild-type and pday mutant retinas combined with zpr1 antibody labeling (red). (C′) Higher magnification of the dorsal, central and ventral ONL of pday mutant retinas, indicated by white rectangles in C. Many TUNEL signals were associated with zpr1 signals (white arrows); however, a few were not associated with zpr1 (white arrowheads). (D) TUNEL (red) of 5-dpf wild-type and pday mutant retinas combined with anti-rhodopsin antibody labeling (green). (D′) Higher magnification of the dorsal, central and ventral ONL of pday mutant retinas, indicated by white rectangles in D. Rhodopsin signals were detected in the peripheral region of dorsal and ventral ONL (yellow arrowheads). TUNEL signals associated with rhodopsin signals were observed only in the peripheral region of the dorsal and ventral ONL (white arrowheads). TUNEL signals were not associated with rhodopsin signals in the central ONL and non-peripheral regions of the dorsal and ventral ONL (white arrows). (E,F) Wild-type and pday mutant retinas with and without the transgene Tg[hsp: mCherry-Bcl2], labelled with zpr1 (E) and zpr3 (F) antibodies. Nuclei were counterstained with Hoechst 33342 (blue). Both zpr1 and zpr3 signals in the ONL were recovered in pday mutant retinas with Tg[hsp: mCherry-Bcl2] (white arrowheads). (F′) Higher magnification of the dorsal ONL indicated by white rectangles in F. Sample sizes are shown in Table S3. Scale bars: 50 µm (A-D); 40 µm (E,F). |
|
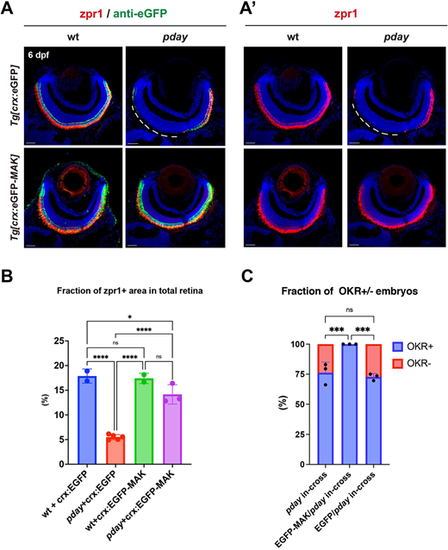
Overexpression of Mak restores photoreceptor survival and visual function of pday mutants. (A,Aʹ) Labeling of wild-type and pday mutant retinas with zpr1 (red, A,Aʹ) and anti-eGFP (green, A) antibodies carrying the transgene Tg[crx:eGFP] or Tg[crx:eGFP-MAK]. Nuclei were counterstained with Hoechst 33342 (blue). zpr1 signals were markedly decreased in the dorso-central region of pday mutant retinas carrying Tg[crx:eGFP] (white dotted lines), but maintained in pday mutant retinas carrying Tg[crx:eGFP-MAK]. Scale bars: 20 µm. (B) The fraction of zpr1-positive area in the total retinal area in wild-type and pday mutants with Tg[crx:eGFP] or Tg[crx:eGFP-MAK]. The fraction was markedly reduced in pday mutants with Tg[crx:eGFP] (red bar), but recovered in pday mutants with Tg[crx:eGFP-MAK] (purple bar), equivalent to that in wild-type with Tg[crx:eGFP] (blue bar) or Tg[crx:eGFP-MAK] (green bar). Bars and lines indicate mean±s.d. Statistical significance was evaluated with ordinary one-way ANOVA and Tukey's multiple comparison tests. ns, not significant; *P<0.0332; ****P<0.0002. (C) The fraction of optokinetic response (OKR)+ (blue) and OKR− (red) embryos relative to total progeny produced by crosses of pday+/− parent fish, pday+/− parent fish carrying Tg[crx:eGFP] and pday+/− parent fish carrying Tg[crx:eGFP-MAK]. 57 of 244 (23.3%) embryos produced by three independent crosses of pday+/− parent fish were negative for OKR. However, 100% of total 257 embryos produced by three independent crosses of pday+/− parent fish carrying Tg[crx:eGFP-MAK] were positive for OKR. 68 of 266 (25.5%) embryos produced by three independent crosses of pday+/− parent fish carrying Tg[crx:eGFP] were negative for OKR. Bars and lines indicate mean±s.d. Statistical significance was evaluated with two-way ANOVA and Tukey's multiple comparison tests. ns, not significant; ***P<0.0002. |
|
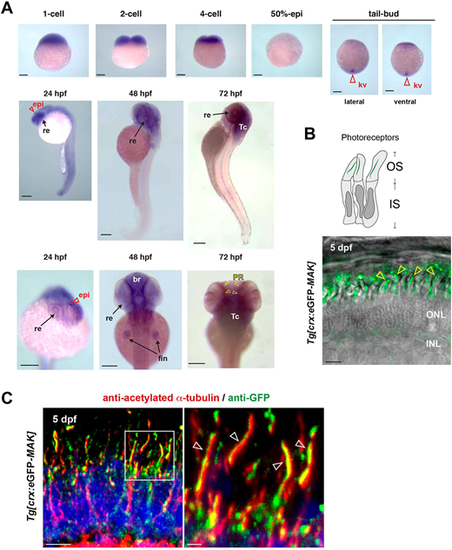
Mak is localized in axonemes of photoreceptor cilia. (A) Whole-mount in situ hybridization of zebrafish embryos with mak RNA probe. mak mRNA expression was observed in Kupffer's vesicles (red open arrowheads) at the tail-bud stage, in the retina (arrows) and epiphysis (red open arrowheads) at 24 hpf, and in the retina, brain and fin (arrows) at 48 hpf. Retinal expression was restricted to the photoreceptor layer (yellow arrowheads) at 72 hpf. kv, Kupffer's vesicle; re, retina; epi, epiphysis; br, brain; fin, pectoral fin; Tc, optic tectum; PR, photoreceptor layer. (B) Confocal live image of Tg[crx: eGFP-MAK] transgenic retina at 5 dpf. eGFP-Mak (green) was localized in the OS of photoreceptors (yellow arrowheads). (C) Labeling of 5-dpf Tg[crx: eGFP-MAK] transgenic retina with anti-acetylated α-tubulin (red) and anti-GFP (green) antibodies. Nuclei were counterstained with Hoechst 33342 (blue). The right panel shows a higher magnification of the square shown in the left panel. eGFP-Mak was localized in the axoneme (white open arrowheads, right panel). Sample sizes are shown in Table S3. Scale bars: 200 µm (A); 5 µm (B, left panel in C); 1 µm (right panel in C). |
|
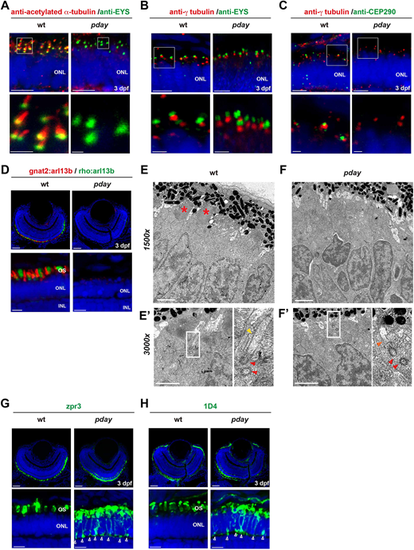
The axoneme is absent in pday mutant photoreceptors. (A-C) Wild-type and pday mutant retinas with anti-acetylated α-tubulin (red) and anti-Eys (green) antibodies (A); anti-γ-tubulin (red) and anti-Eys (green) antibodies (B); and anti-γ-tubulin (red) and anti-Cep290 (green) antibodies (C). (D) Wild-type and pday mutant retinas carrying the transgenes Tg[gnat2: arl13b-tdTomato] (red) and Tg[rho: ail13b-eGFP] (green). (E-Fʹ) Electron microscopy analysis of wild-type (E) and pday mutant (F) photoreceptors at 3 dpf. The bottom-left panels show a higher magnification (3000×) of the apical region of photoreceptors in wild-type siblings (E′) and pday mutants (F′). The bottom-right panels are enlarged images of the rectangular region shown in the bottom left panels. Red asterisks indicate the OS. Red and yellow arrowheads indicate the basal body and ciliary axoneme, respectively. The orange arrowhead indicates the short ciliary extension from the basal body observed in pday mutants. (G,H) Labeling of wild-type and pday mutant retinas with zpr3 (G) and 1D4 (H) antibodies. The open arrowheads indicate ectopic distribution of zpr3 and 1D4 signals in the plasma membrane including the synaptic area in pday mutants. In A-D,G,H, nuclei were counterstained with Hoechst 33342 (blue). In A-C, except for the mutant image in B, the bottom panels show a higher magnification of the ONL shown in the top panels (white boxes). Sample sizes are shown in Table S3. Scale bars: 5 µm (top panels of A-C; bottom panels of D,G,H); 1 µm (bottom panels of A-C; E-F′); 20 µm (top panels of D,G,H). |
|
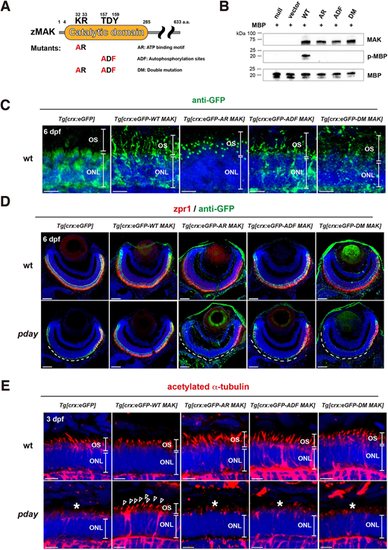
Mak kinase activity is required for Mak protein localization in the ciliary axoneme, axoneme formation and photoreceptor maintenance. (A) A scheme of generation of three types of Mak kinase-dead mutants: AR Mak, ADF Mak and DM Mak. Zebrafish Mak has a catalytic domain, which contains an ATP-binding motif, KR, and an autophosphorylation site, TDY. AR Mak and ADF Mak were generated by conversion of KR and TDY into AR and ADF, respectively. DM Mak has both the AR and ADF mutations. (B) In vitro kinase assay of Mak using a substrate, MBP. Only wild-type Mak phosphorylated MBP. The number of blots is shown in Table S3. (C) Labeling of 6-dpf wild-type ONL carrying the transgene Tg[crx:eGFP], Tg[crx:eGFP-WT MAK], Tg[crx:eGFP-AR MAK], Tg[crx:eGFP-ADF MAK] or Tg[crx:eGFP-DM MAK] with anti-GFP antibody (green). (D) Labeling of 6-dpf wild-type and pday mutant retinas carrying the transgene Tg[crx:eGFP], Tg[crx:eGFP-WT MAK], Tg[crx:eGFP-AR MAK], Tg[crx:eGFP-ADF MAK] or Tg[crx:eGFP-DM MAK] with zpr1 (red) and anti-GFP (green) antibodies. zpr1-positive photoreceptors were degenerated in dorso-central retinas of pday mutants carrying Tg[crx:eGFP] (white dotted line). However, photoreceptor degeneration was rescued only in pday mutants carrying Tg[crx:eGFP-WT MAK], but not in pday mutants carrying kinase-dead mutant transgenes (white dotted line). Images of wild-type and pday mutant retinas carrying the transgenes Tg[crx:eGFP] and Tg[crx:eGFP-WT MAK] are serial sections of the same retinas shown in Fig. 4A. (E) Labeling of 3-dpf wild-type and pday mutant ONL carrying the transgene Tg[crx:eGFP], Tg[crx:eGFP-WT MAK], Tg[crx:eGFP-AR MAK], Tg[crx:eGFP-ADF MAK] or Tg[crx:eGFP-DM MAK] with anti-acetylated α-tubulin antibody (red). Axoneme formation defects were rescued only in pday mutants carrying Tg[crx:eGFP-WT MAK] (arrowheads), but not in pday mutants carrying kinase-dead mutant transgenes (asterisks). In C-E, nuclei were counterstained with Hoechst 33342 (blue). Sample sizes are shown in Table S3. Scale bars: 5 µm (C,E); 40 µm (D). |
|
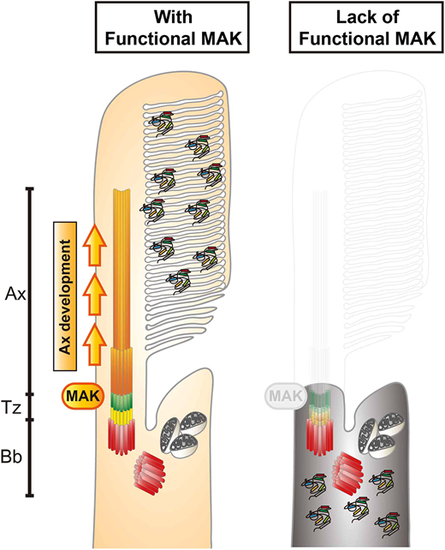
Mak is essential for ciliary axoneme formation in zebrafish photoreceptor ciliogenesis. Phenotypes of zebrafish mak mutant photoreceptors. In the absence of Mak, the axoneme (orange) does not form and the transition zone (green) is affected, resulting in failure to form the OS and to transport phototransduction molecules into the OS. Ax, axoneme; Bb, basal body; Tz, transition zone. |