- Title
-
A deep learning framework for quantitative analysis of actin microridges
- Authors
- Bhavna, R., Sonawane, M.
- Source
- Full text @ NPJ Syst Biol Appl
|
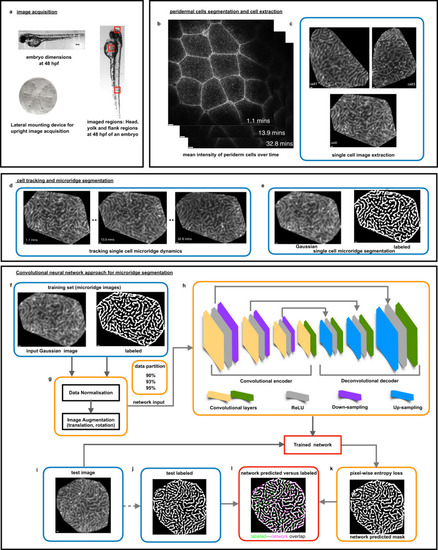
Live imaging, image processing pipeline for a neural network approach for microridge segmentation. |
|
Trained network selection based on predicted accuracy versus network hyperparameters and visual inspection of pixel-wise segmentation. |
|
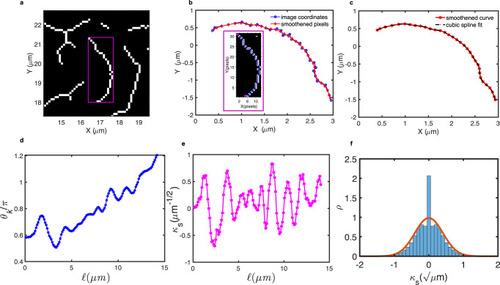
Estimation of persistence length for in vivo microridges. |
|
Population level comparison of cell patterns from yolk versus flank regions. Example of a network segmented |
|
Actin clusters within the microridges exhibit positional fluctuations. |