- Title
-
Antiangiogenic potential of Lepista nuda extract suppressing MAPK/p38 signaling-mediated developmental angiogenesis in zebrafish and HUVECs
- Authors
- Deshmukh, D., Hsu, Y.F., Chiu, C.C., Jadhao, M., Hsu, S.C.N., Hu, S.Y., Yang, S.H., Liu, W.
- Source
- Full text @ Biomed. Pharmacother.
|
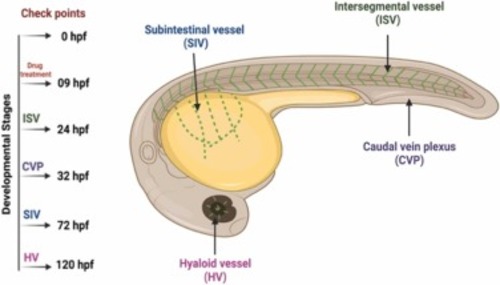
Fig. 1. Representative image of zebrafish embryos and respective checkpoints to observe vascular system development. Developmental angiogenesis was observed through analysis of ISV formation at 24 hpf, CVP at 32 hpf, SIV at 72 hpf, and HV at 2.5–6 dpf. |
|
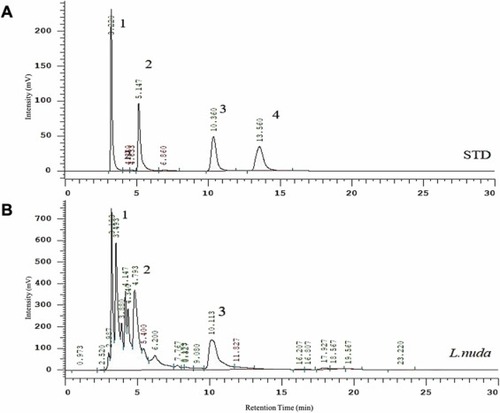
Fig. 2. Representative results of HPLC analysis of L. nuda extract. (A) HPLC chromatogram of a mixture of 4 bioactive components as standards and identified peaks represented as 1 (ergothioneine), 2 (eritadenine), 3 (adenosine), and 4 (cordycepin). (B) HPLC chromatogram of the L. nuda water extract shows a peak relevant to ergothioneine (peak 1), eritadenine (peak 2), and adenosine (peak 3). |
|
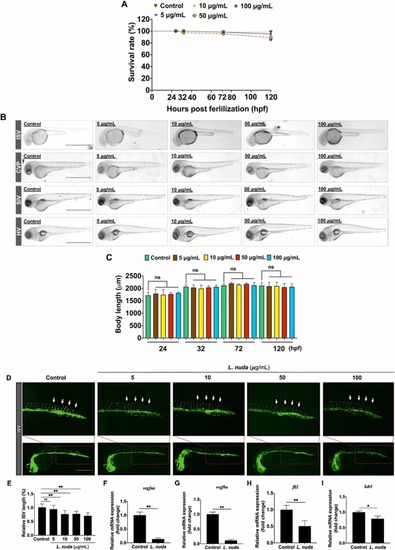
Fig. 3. Representative and quantitative results of L. nuda extract treatment on zebrafish embryo development and inhibition of ISV growth. (A) The relative survival rate of zebrafish embryos showed no cytotoxicity of L. nuda (5–100 μg/mL) treatment (24–120 hpf). (B, C) The body length of zebrafish embryos was measured after L. nuda (5–100 μg/mL) treatment. (D) Lateral assessment of Tg (fli1: EGFP) zebrafish embryos at 24 h postfertilization (hpf) revealed vessel destruction following L. nuda treatment. (E) Relative ISV length was significantly reduced after L. nuda treatment. The white arrow indicates the ISV location. (F-I) The depletion of mRNA levels of vegfaa, vegfba, kdrl, and flt1 following L. nuda treatment (10 μg/mL) as analyzed by qPCR. * p < 0.05 and * * p < 0.001; Scale bar = 100 µm. |
|
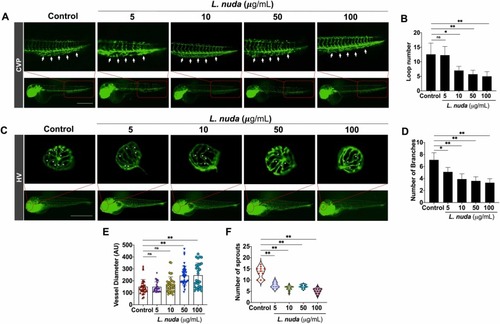
Fig. 4. : Representative and quantitative results of CVP and HV development and the impact of L. nuda extract treatment. (A) The CVP development evaluation shows inhibition of CVP growth and defective and unorganized loops observed following L. nuda treatment. White arrows indicate the locations of the CVP loops. (B) Quantitative results show that L. nuda treatment significantly reduced the number of CVP loops compared to the untreated/control group. (C) HV development evaluated by fluorescence imaging showed a significant effect of L. nuda treatment. White asterisk indicates HV branching. (D-F) Quantification of HV branching, diameter, and sprouting showed significant changes in control and L. nuda (5–100 μg/mL)-treated embryos at 6 dpf. * p < 0.05, * * p < 0.001; Scale bar = 100 µm. |
|
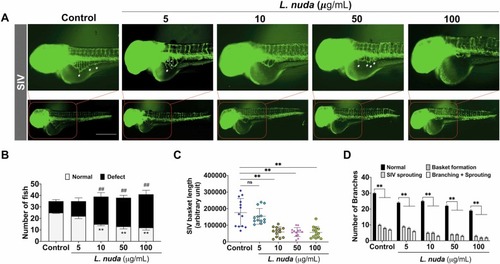
Fig. 5. : Quantitative and representative results of L. nuda extract treatment-mediated SIV inhibition. (A) The representative results of SIV development following L. nuda extract treatment showed a delay in SIV growth in zebrafish embryos. L. nuda extract (5–100 μg/mL) led to the depletion of SIV sprouting, delayed basket formation, and defects in basket growth. White asterisks and hash indicate normal SIV growth, sprouting, branching, and branching + sprouting. (B) Quantitative results of the number of embryos showing normal and defective SIV development. Control (n = 30) and L. nuda extract treatment (n = 30). (C, D) Quantitative results of SIV growth, sprouting, branching, and branching + sprouting observed in individual embryos in control and L. nuda extract-treated embryos observed at 72 hpf. * p < 0.05, ##, * * p < 0.001. Scale bar = 100 µm. |
|
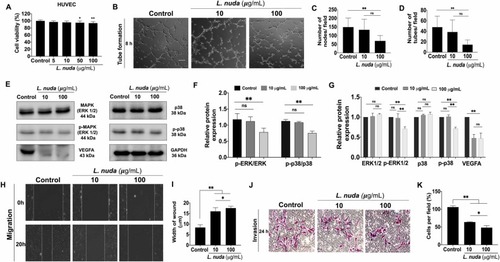
Fig. 6. Quantitative and representative results of the impact of L. nuda extract treatment on HUVEC tube formation, migration, and invasion. (A) Cell viability evaluation showed slight cytotoxicity of L. nuda extract (0, 5, 10, 50, and 100 µg/mL) for 24 h. (B) Evaluation of tube formation following 8 h of L. nuda extract (10 and 100 µg/mL) treatment showed significant inhibition at 100 µg/mL. (C) Quantification of the total number of tubes formed showed a significant decline in the 100 µg/mL L. nuda extract treatment group. (D) Quantification of the total number of nodes formed showed inhibition by 45% following L. nuda extract treatment (100 µg/mL). (E-G) Representative and qualitative results of immunoblotting showed downregulation of MAPK/p38/VEGF signaling markers following L. nuda extract (10 and 100 µg/mL) treatment. Three independent experiments were performed. (H, I) Wound healing assay results showed significant inhibition of HUVEC migration in the L. nuda (10 and 100 μg/mL) treatment groups. (J, K) Transwell invasion assays showed that L. nuda (10 and 100 μg/mL) treatment significantly inhibited HUVEC invasion. * p < 0.05, * * p < 0.001. |
|
Fig. 7. Quantitative results of mRNA expression changes following L. nuda extract treatment in HUVECs by qPCR. (A-C) L. nuda extract treatment (100 µg/mL for 24 h) reduced the mRNA levels of the growth factors fgf, ang2, and vegfa. (D-F) Inhibition of tie2, vegfr, and eng mRNA expression following L. nuda extract treatment (100 µg/mL for 24 h). (G-H) Depletion of mmp1 and mmp2 mRNA levels. (I-L) Inhibition of the mRNA expression of secretory and inflammatory cytokines (il-1α, il −1β, il −6, and tnf-α). * p < 0.05, * * p < 0.001. |
|
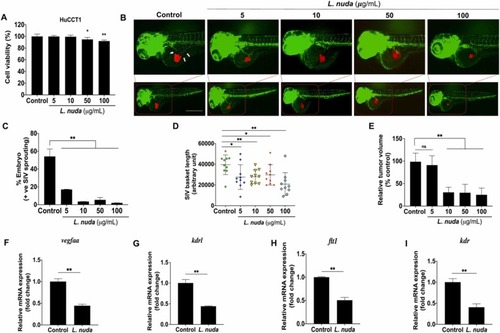
Fig. 8. Representative and quantitative results of the in vivo zebrafish xenograft angiogenesis assay. (A) Cell viability evaluation showed slightly significant cytotoxicity of L. nuda extract (0, 5, 10, 50, and 100 µg/mL) for 24 h. (B) Zebrafish xenograft assays showed that L. nuda extract-treated HuCCT1 cells inhibited SIV sprouting in Tg (fli1: EGFP) zebrafish embryos at 24 hpi. Red fluorescence: DiI-stained HuCCT1 cells; green fluorescence: the vascular network of the zebrafish embryo. White arrows indicate the location of SIV sprouting. White arrow: SIV sprouting. (C-D) Quantitative results of angiogenesis in zebrafish embryos showing SIV inhibition (n = 50) and inhibition of SIV basket length. (E) Quantitative results of in vivo tumor proliferation show depletion of tumor mass following L. nuda extract treatment. (F-I) qPCR results show that L. nuda extract treatment inhibits the mRNA expression of vegfaa, kdrl, flt1and, kdr. Scale bar = 100 µm. * p < 0.05, * * p < 0.001. |
|
Fig. 9. Schematic representation of the antiangiogenic molecular mechanism of the L. nuda extract. |