- Title
-
Exploring Macrophage-Dependent Wound Regeneration During Mycobacterial Infection in Zebrafish
- Authors
- Bohaud, C., Johansen, M.D., Varga, B., Contreras-Lopez, R., Barthelaix, A., Hamela, C., Sapède, D., Cloitre, T., Gergely, C., Jorgensen, C., Kremer, L., Djouad, F.
- Source
- Full text @ Front Immunol
|
|
|
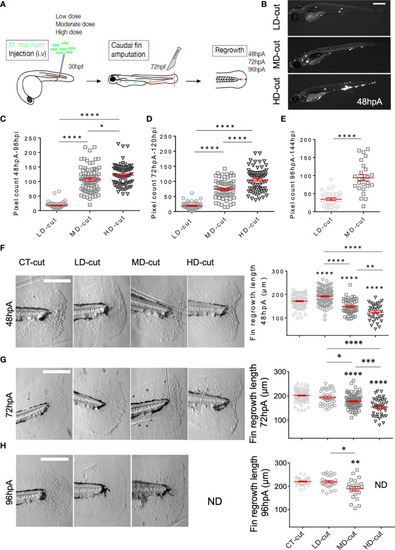
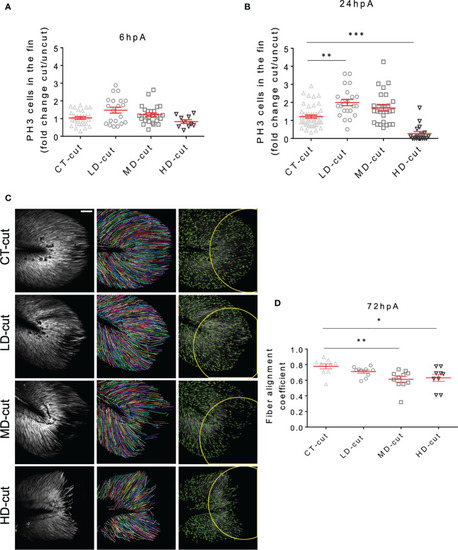
Infection influences cell proliferation, and structure of collagen fibers in the regenerated caudal fin. |
|
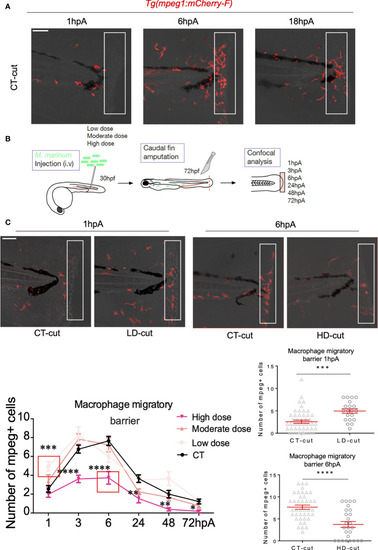
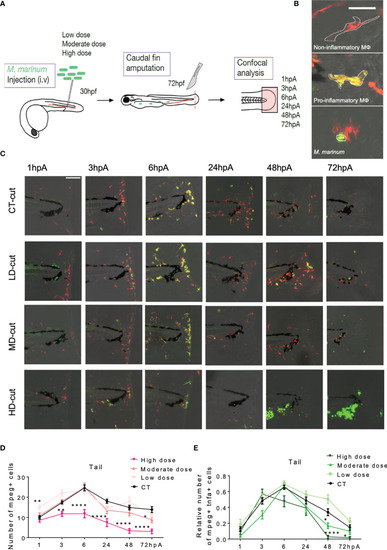
Establishment of the macrophage barrier in the regenerated caudal fin is influenced by the infection. |
|
|
|
|
|
A model describing the macrophage response during the caudal fin regeneration of zebrafish larvae infected with |