- Title
-
Electrophysiological and pharmacological characterization of spreading depolarization in the adult zebrafish tectum
- Authors
- Terai, H., Gwedela, M.N.V., Kawakami, K., Aizawa, H.
- Source
- Full text @ J. Neurophysiol.
|
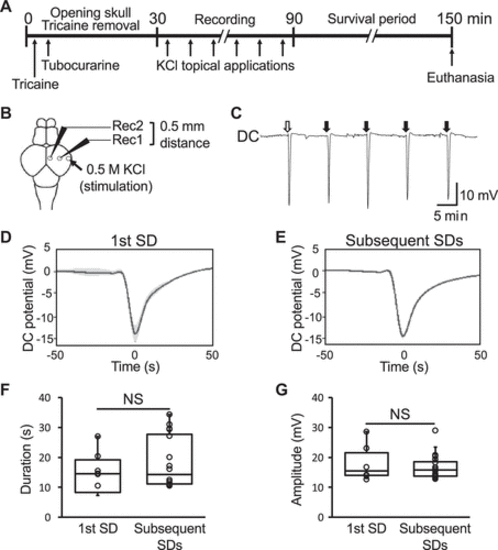
Figure 1.Direct current (DC) potential shift in the adult zebrafish tectum upon chemical stimulation. A: a schematic of the experimental electrophysiological recording process of the extracellular potential in the adult zebrafish tectum. B: a schematic dorsal view of the adult zebrafish brain showing the position of the recording electrodes with an interelectrode distance of 0.5 mm (Rec1 and Rec2) and the KCl stimulation site (arrow). C: a representative trace of the DC extracellular potential recorded in the tectum. The lateral side of the tectum was stimulated every 10 min as indicated by the white (first spreading depolarization, SD) and black (subsequent SDs) arrows. D and E: line plots of the DC potential shifts associated with KCl stimulation showing the mean of the first (D, n = 6 SDs) and subsequent (E, n = 85 SDs) SDs recorded in the sessions. Data are presented as the means (solid lines) ± standard error (SE, shaded area). Bar graphs with dot-whisker plots of the duration at half of the maximal amplitude (F) and amplitude (G) of the first and subsequent SDs. NS, not significant. |
|
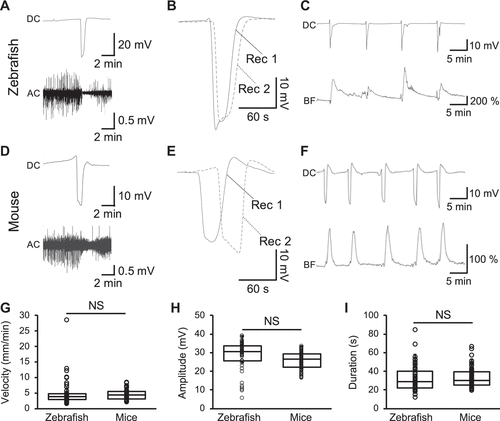
Figure 2.Concurrent changes in alternating current (AC) potential and hemodynamics of the zebrafish tectum during spreading depolarization (SD). Representative traces of the direct current (DC, top) and AC (bottom) components of the extracellular potential recorded in the zebrafish tectum (A) and the mouse cortex (D) after KCl stimulation. Representative traces of the DC potential recorded with two electrodes (Rec1 and Rec2) in the zebrafish tectum (B, interelectrode distance: 0.5 mm) and mouse cortex (E, interelectrode distance: 2 mm). Note the delayed negative peak in Rec2, which is further from the stimulation site than Rec1. Representative traces of the DC potential (top) and local blood flow (bottom) in the zebrafish tectum (C) and the mouse cortex (F) upon KCl stimulation. Bar graphs with dot-whisker plots of the velocity (G), amplitude (H), and duration at the half of the maximal amplitude (I) of SD recorded in the zebrafish tectum and the mouse cortex. NS, not significant; n = 8 fish, n = 4 mice. |
|
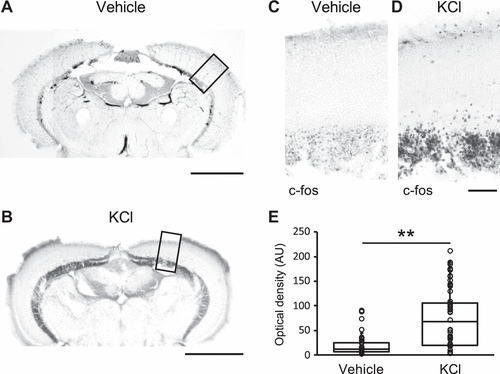
Figure 3.Neuronal activation associated with spreading depolarization (SD) as revealed by immediate early gene c-fos expression. Coronal sections of the tectum treated with PBS as the vehicle (A) or KCl (B) showing the expression of the immediate early gene-c-fos protein (black). Scale: 500 µm. C and D: magnified images of A and B respectively. Scale: 50 µm. E: bar graphs with dot-whisker plots of the signal intensity measured by optical density (arbitrary unit) of c-fos protein expression in the tectum of the zebrafish treated with vehicle (n = 4), or KCl (n = 7). **P < 0.01. |
|
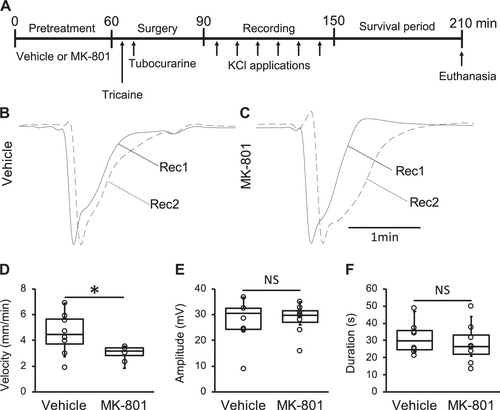
Figure 4.An N-methyl-d-aspartate (NMDA) receptor antagonist attenuated spreading depolarization (SD) propagation in the zebrafish tectum. A: a schematic of the experimental process used to assess the effect of MK-801 (NMDA receptor antagonist) on SD in the zebrafish tectum. Representative traces of direct current (DC) potentials at the proximal (Rec1, solid lines) and distal (Rec2, dashed lines) electrodes in the optic tectum of the zebrafish pretreated with vehicle (B) and MK-801 (C). Bar graphs with dot-whisker plots of SD velocity (D), amplitude (E), and duration (F) observed in zebrafish pretreated with vehicle (n = 8) and MK-801 (n = 4). *P < 0.05. NS, not significant. |
|
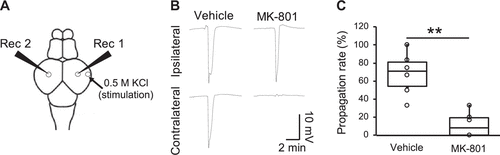
Figure 5.MK-801 suppressed spreading depolarization (SD) propagation across hemispheres. A: a schematic dorsal view of the zebrafish brain showing the positions of the ipsilateral (Rec1) or contralateral (Rec2) electrodes. B: representative traces of DC potentials recorded in the tectum ipsilateral (top) and contralateral (bottom) side of the zebrafish pretreated with vehicle (left) or MK-801 (right). C: bar graphs with dot-whisker plots of the SD propagation rate from the stimulated hemisphere to the contralateral hemisphere of zebrafish pretreated with vehicle or MK-801 (n = 6, respectively). **P < 0.01. |