- Title
-
Probing the Effects of the FGFR-Inhibitor Derazantinib on Vascular Development in Zebrafish Embryos
- Authors
- Kotini, M.P., Bachmann, F., Spickermann, J., McSheehy, P.M., Affolter, M.
- Source
- Full text @ Pharmaceuticals (Basel)
|
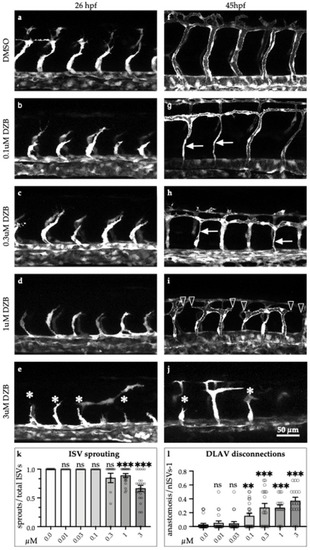
Derazantinib inhibits vascular development in vivo in a dose-dependent manner. Confocal images of GFP+ blood vessels in the trunk of |
|
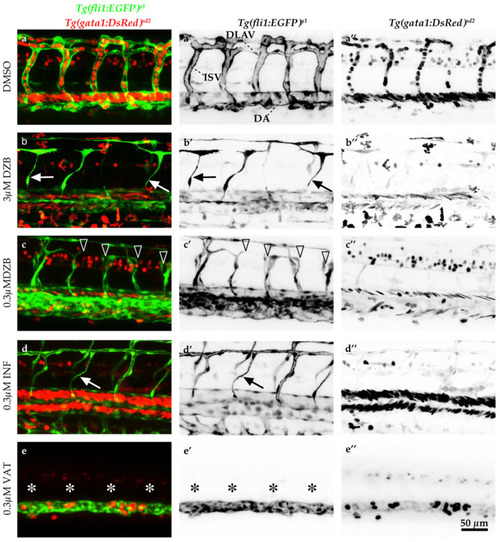
Comparisons of derazantinib, infigratinib and vatalanib blood vessel function. Confocal images of GFP+ blood vessels and DsRed+ erythrocytes in the trunk of |
|
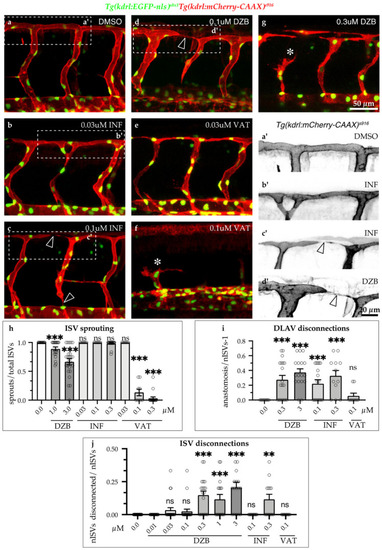
Comparisons of derazantinib, infigratinib and vatalanib during vascular development. Confocal images of GFP+ endothelial cell nuclei and mCherry+ endothelial cell membranes in |
|
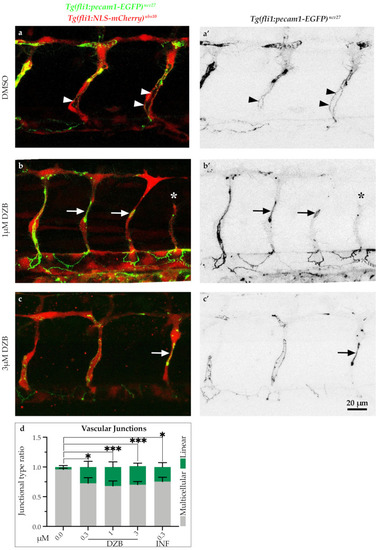
Derazantinib interferes with endothelial cell junctions. Confocal images of GFP+ endothelial cell junctions and mCherry+ endothelial cell nuclei in |
|
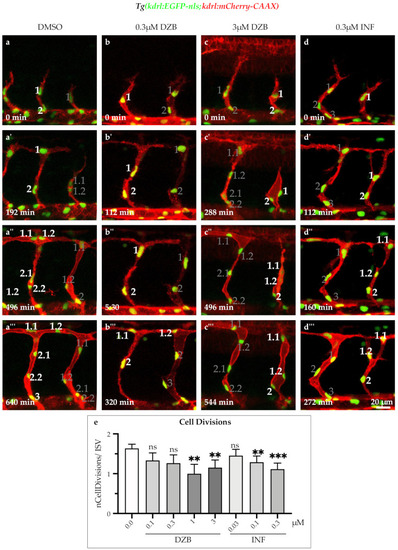
Derazantinib and infigratinib inhibit endothelial cell cycle. Time-lapse images of sprouting ISVs of GFP+ endothelial cell nuclei and mCherry+ endothelial cell membranes in |