- Title
-
Naringin attenuates cisplatin- and aminoglycoside-induced hair cell injury in the zebrafish lateral line via multiple pathways
- Authors
- Li, M., Liu, J., Liu, D., Duan, X., Zhang, Q., Wang, D., Zheng, Q., Bai, X., Lu, Z.
- Source
- Full text @ J. Cell. Mol. Med.
|
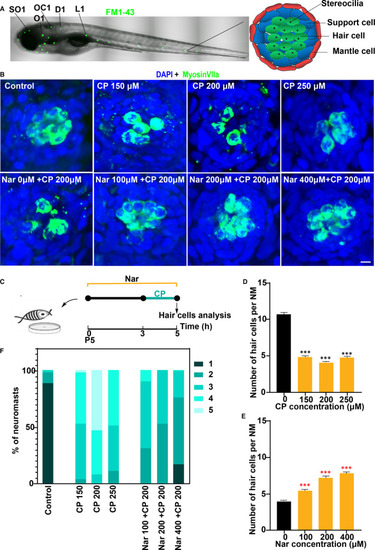
Nar protects against cisplatin ototoxicity. (A) Confocal image of a 5 d post‐fertilization (dpf) zebrafish. The neuromasts analysed in this experiment are indicated by black lines: SO1(supraorbital 1), O1 (otic 1), OC1(occipital 1), D1(dorsal trunk 1) and L1(lateral 1). Schematic top view of a 5 dpf neuromast showing the different cell types is shown in the right. (B) Animals were fixed and immunostained for MyosinVIIa (green) and DAPI. 5dpf zebrafish were incubated with 0, 150, 200 and 250 μmol/L cisplatin (CP) for 2 h, or pre‐treated with 0 µmol/L to 400 µmol/L of Naringin(Nar) for 3 h and then co‐treated with Nar and CP (150 µmol/L) for 2 h. (C) Diagram of the assay for B. (D and E) Quantification of the number of hair cells (MyosinVIIa+) per neuromast after the different treatments represented as mean ± SEM (n = 10). Control animals were exposed to vehicle alone (DMSO). *** |
|
Nar protects against gentamicin ototoxicity. (A) Animals were fixed and immunostained for MyosinVIIa (green) and DAPI. 5 dpf zebrafish were incubated with 0 μmol/L to 150 μmol/L GM for 3 h or pre‐treated with 0 µmol/L to 400 µmol/L of Nar for 3 h and then co‐treated with Nar and GM (100 µmol/L) for 3 h. (B) Diagram of the assay for A. (C and D) Quantification of the number of hair cells (MyosinVIIa+) per neuromast after the different treatments represented as mean ± SEM (n = 10). Control animals were exposed to vehicle alone (DMSO). ** |
|
Nar protects against neomycin ototoxicity. (A) Animals were fixed and immunostained for MyosinVIIa (green), and DAPI. 5 dpf zebrafish were incubated with 0 μmol/L to 150 μmol/L Neo for 1 h, or pre‐treated with 0 µmol/L to 400 µmol/L of Nar for 3 h and then co‐treated with Nar and Neo (100 µmol/L) for 3 h. (B) Diagram of the assay for A. (C and D) Quantification of the number of hair cells (MyosinVIIa+) per neuromast after the different treatments represented as mean ± SEM (n = 10). Control animals were exposed to vehicle alone (DMSO). *** |
|
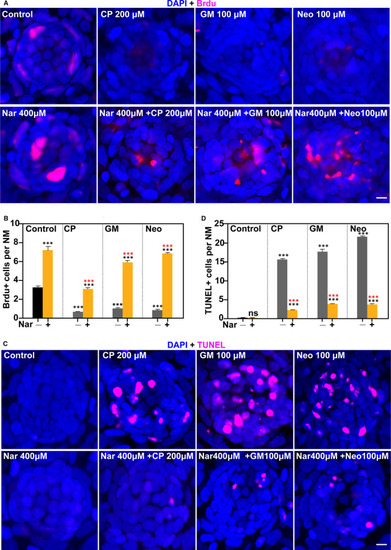
Nar promotes cell proliferation and protects against ototoxin‐induced cell death in neuromasts. (A) Proliferation assays were performed in 5 dpf zebrafish in the presence/absence of Nar (400 µmol/L) and the corresponding ototoxin. Animals were fixed and immunostained for BrdU (pink) and DAPI. (B) The numbers of proliferated cells (BrdU+) per neuromast were calculated for each treatment and represented as mean ± SEM (n = 10). *** |
|
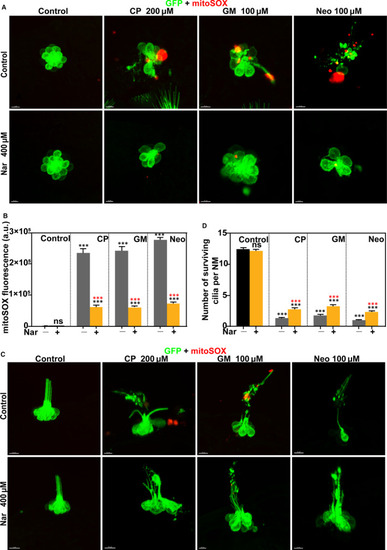
Protective effect of naringin against Mitochondrial oxidative stress and cilia damage. Zebrafish were treated with different concentrations of ototoxic drugs with or without Nar. Control animals were exposed to vehicle alone (DMSO). Still images of live brn3c: GFP hair cells (green) loaded with the live fluorescent indicator mitoSOX (red). (A) The top view of hair cell clusters to show the morphology of hair cells. (B) The mitoSOX fluorescent intensity per neuromast was calculated for each treatment and represented as mean ± SEM. *** |
|
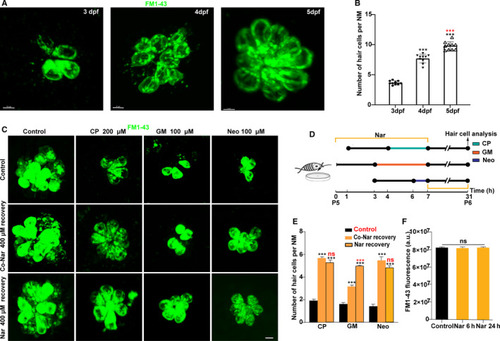
Naringin promotes the regeneration of functional hair cells. A, Still imaging of hair cells of zebrafish larvae 3, 4 and 5 d after FM1‐43 labelled (green) fertilization. B, Scatter plot of FM1‐43 labelled hair cell count at each time‐point (*** |
|
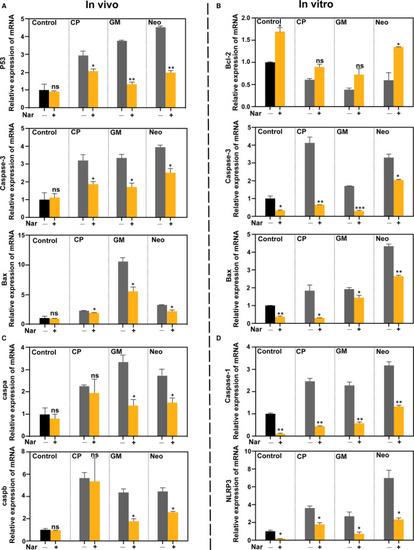
Naringin attenuates the activation of the apoptotic‐ and pyroptosis‐related pathway in vitro and in vivo. (A and C) Real‐time PCR analysis of the mRNA levels of apoptotic ( |