- Title
-
Modular Detection of GFP-Labeled Proteins for Rapid Screening by Electron Microscopy in Cells and Organisms
- Authors
- Ariotti, N., Hall, T.E., Rae, J., Ferguson, C., McMahon, K.A., Martel, N., Webb, R.E., Webb, R.I., Teasdale, R.D., Parton, R.G.
- Source
- Full text @ Dev. Cell
|
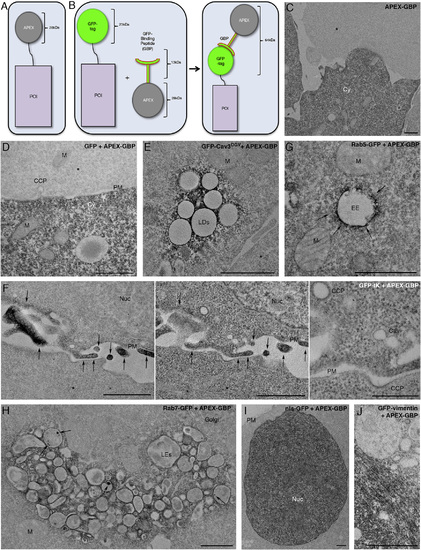
APEX-GBP Correctly Localizes to GFP-Tagged Subcellular Markers in BHK Cells (A) Schematic of conventional APEX-tagging. (B) Schematic of modular APEX tagging consisting of an APEX tag conjugated via linker domain to GFP-binding peptide. (C) APEX-GBP expression alone is soluble and present only in the cytoplasm of transfected cells. (D) APEX-GBP when co-transfected with GFP results in a similar distribution of DAB reaction product to APEX-GBP alone. (E) GFP-Cav3DGV is known to localize to lipid droplets; DAB reaction product was restricted to the surface of morphologically identifiable lipid droplets. (F) Co-transfection of GFP-tK translocates the DAB reaction product to the PM. Left: unstained, central. Right: post-stained. Arrows indicate areas of enriched density at the PM. Post-staining demonstrates the quality of the preservation as this method improves the contrast of membranes but reduces the signal-to-noise ratio of the APEX-GBP. Post-staining reveals clathrin coated pits and caveolae at the surface of BHK cells. (G) Rab5-GFP overexpression with APEX-GBP results in prominent endosomal staining that is tightly opposed to membrane: highlighted by arrows. (H) Rab7-GFP overexpression with APEX-GBP results in significant DAB reaction at later-stage endosomes within transfected cells. Arrows indicate the accumulation of Rab7-GFP and APEX-GBP within endosomes. (I) nls-GFP expression with APEX-GBP results in reaction product exclusively in the nucleus of transfected cells. (J) GFP-vimentin was co-transfected with APEX-GBP and demonstrated significant electron density in perinuclear regions and in bundled fibril extensions into the cytoplasm. Untransfected adjacent cells without reaction product, indicating the reaction product is specific. Cy, cytoplasm; Cav, Caveolae; CCP, clathrin-coated pit; M, mitochondria; PM, plasma membrane; LDs, lipid droplets; EE, early endosome; LEs, late endosomes; Nuc, nucleus; IFs, intermediate filaments. Scale bars represent 1 µm. The above localizations were confirmed by confocal microscopy; see Figure S1. |
|
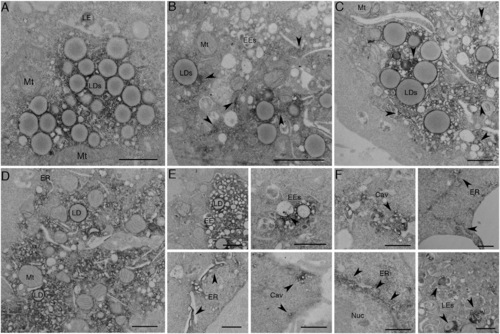
APEX-GBP Allows for Time-Resolved Protein Redistribution (A–F) BHK cells were transfected and serum starved for (A) 0 min, (B) 30 min, (C) 1 hr, (D) 2 hr, (E) 4 hr, and (F) 8 hr to track the redistribution of GFP-CavDGV by APEX-GBP in a time-resolved manner. Arrows highlight regions where electron density has re-distributed from lipid droplets to other intracellular organelles. LD, lipid droplet; Mt, mitochondria; EE, early endosome; LE, late endosome; ER, endoplasmic reticulum; Nuc, Nucleus; Cav, Caveolae. Scale bars represent 500 nm. The above localizations were confirmed by confocal microscopy; see Figure S2. |
|
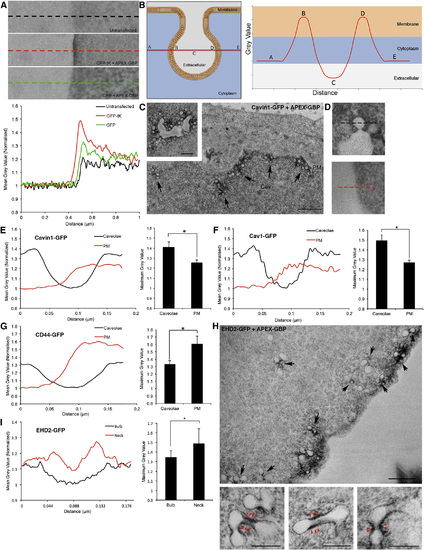
Quantitative Assessment of Caveolar Coat Protein Enrichment at Caveolae Compared to the Bulk Plasma Membrane (A) Top: linescan from extracellular to intracellular regions were taken perpendicular to the PM and demonstrate the quantitative contrast differences between untransfected cells, GFP-tK + APEX-GBP and GFP + APEX-GBP. Bottom: the profiles of the linescan indicate density enrichment at the PM to cells expressing GFP-tK and the cytoplasm in cell expressing GFP. (B) Graphical schematic of linescans performed across caveolae at the region of the caveolar bulb with greatest diameter such that the membrane is spanned twice and parallel to the bulk plasma membrane. (C) APEX-GBP mediated localization of Cavin1-GFP to caveolae at the PM of BHK cells. Arrows indicate caveolae at the PM positive for Cavin1-GFP and APEX-GBP. Cav, caveolae. Scale bar represents 500 nm; inset, 200 nm. (D) Top: high-magnification image of a linescan performed across caveolae positive for Cavin1-GFP and APEX-GBP. Bottom: high-magnification image of a linescan perpendicular to the PM from the same cell. (E) Left: quantification comparing average gray values between bulk PM and caveolae from six different regions of the same cell expressing Cavin1-GFP + APEX-GBP to gain an average value. Multiple cells were quantified from multiple independent repeats (n = 4). Right: quantification of average maximum gray values. Statistical significance was determined using two-tailed t tests (p = 0.045); error bars represent SEM. (F and G) Caveolin-1-GFP (Cav1) + APEX-GBP (p = 0.014) demonstrated a similar enrichment of electron density at the sites of caveolae compared to the bulk PM however CD44-GFP + APEX-GBP (p = 0.05) was significantly enriched at the PM and excluded from caveolae. (H) EHD2-GFP is enriched at caveolae on the PM; however, the electron density generated by the DAB reaction and APEX-GBP binding suggests EHD2 is excluded from the caveolar bulb and specifically enriched at the neck of caveolae. Black arrows highlight caveolae with enriched electron density at the neck of caveolae and red arrowheads denote enriched electron density at the caveola neck at higher magnification. Scale bars at lower magnification represent 500 nm. Scale bars for insets represent 200 nm. (I) Left: quantitative linescans comparing average gray values between the caveolar bulb and the caveolar neck region in cells expressing EHD2-GFP (n = 3). Bottom right: quantification of average maximum gray values. Statistical significance was determined using two tailed t tests (p = 0.0083); error bars represent the SD of the mean. |
|
Rapid Screening of a GFP-Tagged Phosphoinositide Probe Library using APEX-GBP Allows for Fast and Reliable Protein Localization (A–H) GFP-PLC-PH, GFP-BTK-PH, GFP-AKT-PH, GFP-TAPP1-PH, GFP-2xFYVEhrs, GFP-OSBP-PH, GFP-2ML1N and GFP-2ML1N (7Q) detected at known subcellular localizations by APEX-GBP co-transfection. Scale bars represent 1 µm. Untransfected adjacent cells without reaction product. Arrowheads represent enriched electron density at the PM, arrows highlight accumulation of reaction product within endosomes. Cy, cytoplasm; PM, plasma membrane; EEs, early endosomes; LE, late endosomes; Golgi, Golgi complex; M, mitochondria; CCP, clathrin-coated pit. The above localizations were confirmed by confocal microscopy; see Figure S3. Comparisons of ultrastructure between modular and directly APEX-tagged PI probes were performed; see Figures S4A–S4C. |
|
Rapid Screening of a GFP-Tagged Phosphoinositide Probe Library using APEX-GBP Allows for Fast and Reliable Protein Localization (A–H) GFP-PLC-PH, GFP-BTK-PH, GFP-AKT-PH, GFP-TAPP1-PH, GFP-2xFYVEhrs, GFP-OSBP-PH, GFP-2ML1N and GFP-2ML1N (7Q) detected at known subcellular localizations by APEX-GBP co-transfection. Scale bars represent 1 µm. Untransfected adjacent cells without reaction product. Arrowheads represent enriched electron density at the PM, arrows highlight accumulation of reaction product within endosomes. Cy, cytoplasm; PM, plasma membrane; EEs, early endosomes; LE, late endosomes; Golgi, Golgi complex; M, mitochondria; CCP, clathrin-coated pit. The above localizations were confirmed by confocal microscopy; see Figure S3. Comparisons of ultrastructure between modular and directly APEX-tagged PI probes were performed; see Figures S4A–S4C. |
|
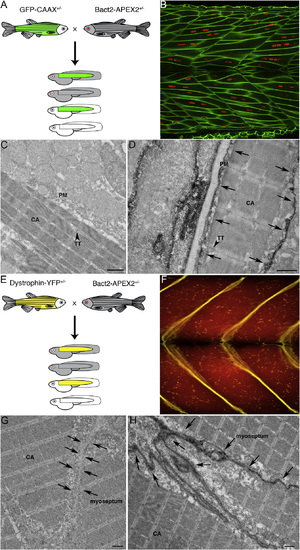
APEX-GBP Can Detect Proteins Expressed at Endogenous Levels In Vivo (A) Schematic of GFP-CAAX zebrafish cross with Bact2-APEX-GBP zebrafish. (B) Confocal image of GFP-CAAX expressing zebrafish trunk muscle demonstrating GFP-CAAX is targeted to the sarcoplasmic reticulum. Green, GFP-CAAX; red, nuclei. (C) The APEX-GBP single transgenic zebrafish fish demonstrates soluble reaction product. (D) Electron micrograph of GFP-CAAX APEX-GBP double transgenic zebrafish muscle showing specific electron density at the PM of cells. Arrows highlight the electron density generated by the osmiophilic DAB reaction product accumulating at the site of the APEX-GBP modular tag and the GFP-CAAX protein in the double transgenic zebrafish line. Scale bars represent 1 µm. (E) Schematic of Dystrophin-YFP zebrafish cross with Bact2-APEX-GBP zebrafish. (F) Confocal image of Dystrophin-YFP expressing zebrafish trunk muscle demonstrating Dystrophin-YFP is targeted to the myoseptum. (G) The APEX-GBP single transgenic zebrafish fish demonstrates soluble reaction product. Arrows highlight the position of the myoseptum. (H) Electron micrograph of Dystrophin-YFP APEX-GBP double transgenic zebrafish muscle showing specific electron density at the myoseptum. Arrows highlight the position of the myoseptum and demonstrate reaction product accumulating at the site of the APEX-GBP modular tag and the Dystrophin-YFP protein in the double transgenic zebrafish line. CA, contractile apparatus; TT, transverse tubules; PM, plasma membrane. Scale bars represent 1 µm. |
|
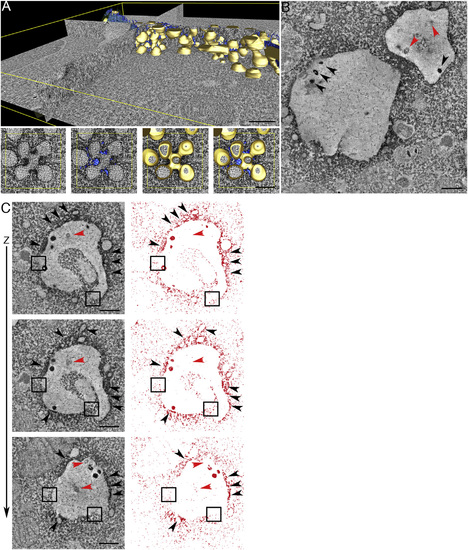
Inducible Control of APEX-GBP Expression In Vivo (A) EM of muscle tissue from a double transgenic non-heat shocked GFP-CAAX zebrafish crossed with the APEX-GBP zebrafish under control of the hsp70I promoter. No electron density can be observed at the surface of muscle cells. Scale bar represents 1 µm. (B) EM of a double transgenic heat-shocked GFP-CAAX zebrafish expressing APEX-GBP (hsp70I promoter). Arrows highlight the electron density generated by the osmiophillic DAB reaction product accumulating at the site of the APEX-GBP tag and the GFP-CAAX protein at the PM of muscle cells. Arrowheads of higher magnification panels demonstrate sensitive detection of the transverse tubule network (observed by fluorescence microscopy in Figure 7B). CA, contractile apparatus; TT, transverse tubules; PM, plasma membrane; Nuc, nucleus. Scale bar represents 1 µm. (C) Backscatter detection of zebrafish muscle tissue demonstrates the electron density generated by the DAB reaction can be observed in the 3view scanning electron microscope. Scale bar represents 1 µm. (D) Aligned image stack and segmentation of the cell surface of a muscle cell from a GFP-CAAX APEX-GBP (hsp70I promoter) zebrafish line demonstrating the discernable PM in three-dimensions (white, PM; blue, nucleus). Refer to Movie S3 for three-dimensional aligned image stack. |
|
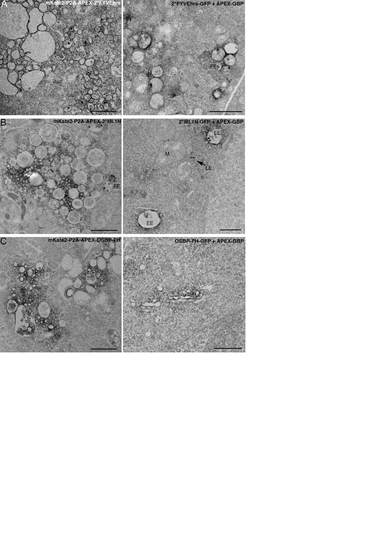
The expression of some directly tagged APEX phosphoinositide probes disrupted normal endomembrane morphology compared to APEX-GBP. A) mKate2-P2A-APEX-2*FYVEhrs expression (left panel) alters early endosome morphology compared to GFP-2*FYVEhrs + APEX-GBP (right panel). B) mKate2-P2AAPEX- 2*ML1N (left panel) expression disrupts late endosome morphology compared to GFP-2*ML1N + APEX-GBP (right panel). C) mKate2-P2A-APEX-OSBP-PH (left panel) expression affects Golgi complex morphology compared to GFP-OSBP-PH + APEX-GBP (right panel). EEs = early endosomes, LE = late endosomes, Golgi = Golgi complex, M = mitochondria. Scale bars = 1 µm. |
Reprinted from Developmental Cell, 35(4), Ariotti, N., Hall, T.E., Rae, J., Ferguson, C., McMahon, K.A., Martel, N., Webb, R.E., Webb, R.I., Teasdale, R.D., Parton, R.G., Modular Detection of GFP-Labeled Proteins for Rapid Screening by Electron Microscopy in Cells and Organisms, 513-25, Copyright (2015) with permission from Elsevier. Full text @ Dev. Cell