- Title
-
A zebrafish model of lethal congenital contracture syndrome 1 reveals Gle1 function in spinal neural precursor survival and motor axon arborization
- Authors
- Jao, L.E., Appel, B., and Wente, S.R.
- Source
- Full text @ Development
|
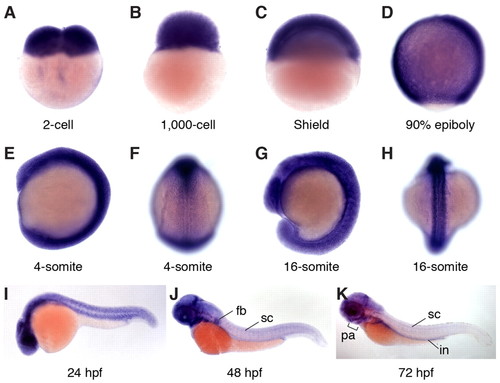
Expression patterns of zebrafish gle1 transcripts. (A-K) Whole-mount RNA in situ hybridization shows that zebrafish gle1 transcripts are maternally deposited and ubiquitously expressed during early development (through 24 hpf). From 48 hpf, gle1 is prominent in the CNS, eyes, pharyngeal arches (pa), fin buds (fb) and intestine (in). F and H show dorsal views; all other panels show lateral views. sc, spinal cord. EXPRESSION / LABELING:
|
|
Morphological phenotypes of gle1–/– mutants. (A-D) Lateral (A,B) and dorsal (C,D) views of wild-type (A,C) and gle1–/– (B,D) zebrafish embryos at 2 dpf. Arrowheads (B,D) mark the smaller head and eyes; arrow (D) marks underdeveloped pectoral fins. (E,F) Acridine Orange-stained 2 dpf wild-type (E) and gle1–/– (F) embryos. Cell death is noted in the head (F, arrow) and spinal cord (F, inset). (G,H) Alcian Blue-stained head cartilages of 5-dpf wild-type (G) and gle1–/– (H) larvae. gle1–/– mutant lacks most viscerocranium but has relatively intact neurocranium. (I,J) Lateral views of 5-dpf wild-type (I) and gle1–/– (J) larvae. Asterisk (J) marks pericardial edema. (K,L) Hematoxylin and Eosin (H&E)-stained transverse sections of wild-type (K) and gle1–/– (L) larvae at 5 dpf. gle1–/– mutant has a smaller spinal cord (L, arrow in inset). Asterisk (L) marks subcutaneous edema; arrowhead (L) marks unfolded intestine. nc, notochord; sc, spinal cord. Scale bars: 500 μm in B,D,F,J; 100 μm in H,L and F inset; 50 μm in L inset. |
|
Motoneuron phenotypes of gle1–/– mutants. (A-D) Confocal images of live wild-type (A,C) and gle1–/– (B,D) zebrafish embryos with motoneurons labeled by mnx1:GFP transgene at 54 hpf (A,B) and 78 hpf (C,D). Compared with wild-type siblings, gle1–/– embryos have fewer motoneurons in the spinal cord (B, bracket), lack rostrally projecting axons (asterisks in wild type, A) around the horizontal myoseptum, and exhibit disorganized axon arbors and ectopic branching (B,D). The phenotypes are exacerbated with age. Images are lateral views of the trunk spinal cord with dorsal to top, anterior to left. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Motoneuron phenotypes of gle1 morphants. (A-F) Confocal images of live control (A,D) and gle1 morphant (B,C,E,F) zebrafish embryos with motoneurons labeled by mnx1:TagRFP-T transgene at 50 hpf (A-C) and 82 hpf (D-F). gle1 morphants were injected with buffer alone (B,E) or with <140 pg in vitro transcribed EGFP-tagged human GLE1b mRNA (C,F). gle1 morphants lack rostrally projecting nerves and show pronounced defects in motor axon arborization (B,E), especially at 82 hpf, when the gle1 morphant also lacks dorsally projecting axons (E, brackets) and aberrations in ventrally projecting axons are particularly evident. Expressing human GLE1 efficiently rescues the arborization defects (C,F). Images are lateral views of the trunk spinal cord with dorsal to top, anterior to left. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
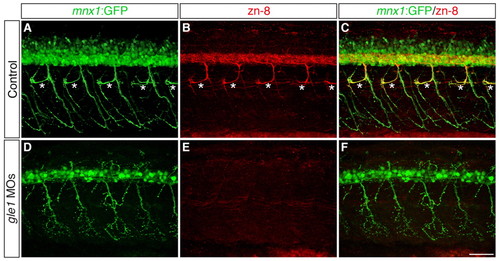
Secondary motoneurons are severely reduced in gle1 morphants. (A-F) Confocal images of control (A-C) and gle1 morphant (D-F) zebrafish embryos with motoneurons labeled by mnx1:GFP transgene and zn-8 (Alcama) antibody to reveal somata and fasciculated axons of secondary motoneurons at 56 hpf. gle1 morphants lose most zn-8 immunoreactivity, including the rostrally projecting nerves (asterisks in the control, A-C), but still retain the primary motoneurons (mnx1:GFP+) (D,F). Images are lateral views of the trunk spinal cord with dorsal to top, anterior to left. Scale bar: 50 μm. |
|
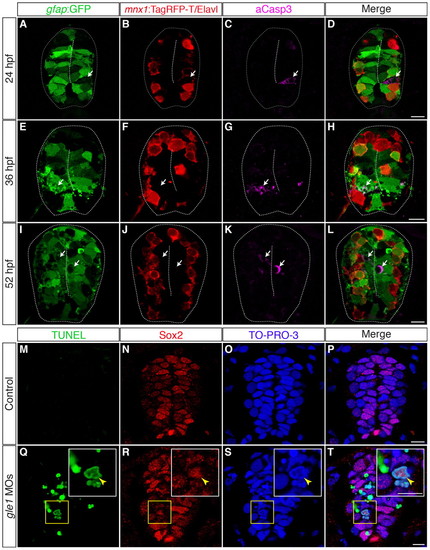
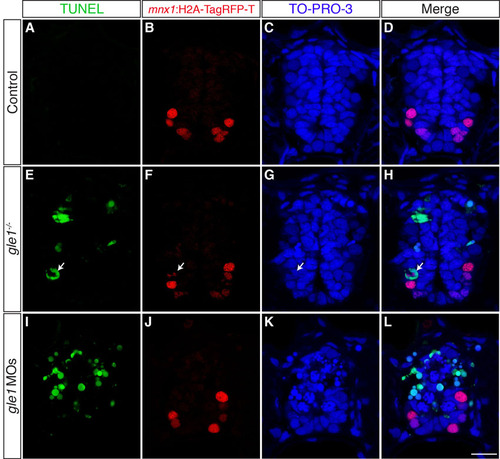
Neural precursors, rather than differentiated neurons, undergo apoptosis in gle1 morphants. (A-L) Transverse cryosections through the spinal cords of gle1 morphants harboring gfap:GFP and mnx1:TagRFP-T transgenes were stained with anti-Elavl and anti-cleaved caspase 3 (aCasp3) antibodies at 24 (A-D), 36 (E-H) and 52 (I-L) hpf. The gfap:GFP transgene reveals the radial glia (A,E,I); anti-Elavl staining reveals newly differentiated neurons with a cytoplasmic signal; the mnx1:TagRFP-T transgene labels motoneurons with both nuclear and cytoplasmic signals (B,F,J); aCasp3 labels apoptotic cells (C,G,K). aCasp3+ cells are often gfap:GFP+ (arrows), located close to the central canal (dashed lines), and/or in close proximity with Elavl+ mnx1:TagRFP-T+ neurons. Elavl+ mnx1:TagRFP-T+ neurons are always negative for aCasp3 (merged channels, D,H,L). (M-T) Transverse cryosections through the spinal cords of control (M-P) and gle1 morphant (Q-T) embryos at 31 hpf were stained for Sox2, followed by TUNEL reactions, to reveal Sox2+ neural precursors (N,R) and TUNEL+ apoptotic cells (M,Q). Nuclei were counterstained with TO-PRO-3 (O,S). Detection of Sox2+ TUNEL+ nuclei (arrowheads) indicates that Sox2+ neural precursors die upon Gle1 depletion. Each image is a single optical section (<1 μm) extracted from the confocal z-stack (dorsal to top). Dashed lines outline spinal cords and central canals. Scale bars: 10 μm in D,H,L; 7.5 μm in P,T. |
|
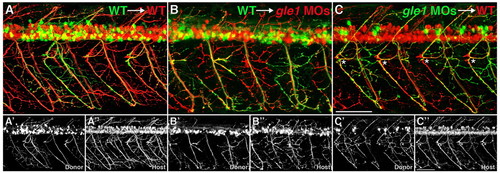
Gle1 acts non-cell-autonomously in motor axon arborization. (A-C3) Confocal images of live chimera embryos at 3 dpf. Cells from either wild-type (A,B) or gle1 morphant (C) donor embryos (mnx1:GFP+) were transplanted into wild-type (A,C) or gle1 morphant (B) hosts (mnx1:TagRFP-T+). Wild-type donor motoneurons extend their axons and follow the aberrant motor axons of the gle1 morphant host (B). Conversely, motoneurons from the gle1 morphant donor extend their axons normally in the wild-type host (C) as in the case with the wild type-to-wild type transplantation (A). Rostrally projecting motor nerves from the gle1 morphant donor are restored in the wild-type environment (asterisks, C). Single-channel images (A2,A3,B2,B3,C2,C3) are shown beneath the merged images to distinctly visualize donor and host axons. Images are lateral views of the trunk spinal cord with dorsal to top, anterior to left. Scale bars: 75 μm. |
|
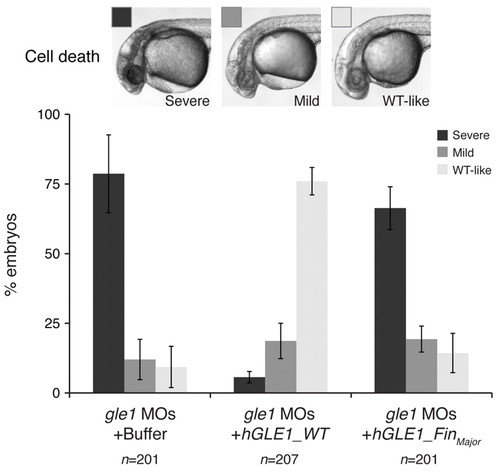
Wild-type human GLE1, but not the FinMajor mutant form, rescues the defects caused by Gle1 depletion. Embryos from gle1hi4161a/+ intercrosses were injected with a mix of two non-overlapping gle1 translation-blocking morpholinos (gle1 MOs). Following MO injection, the embryos were injected with buffer alone (gle1 MOs + Buffer) or with <140 pg of in vitro transcribed human GLE1 mRNA (wild type, gle1 MOs + hGLE1_WT; or the FinMajor mutant form, gle1 MOs + hGLE1_FinMajor) at the one-cell stage. The extent of head cell death – categorized as severe, mild or wild-type-like (WT-like) – was scored at 32 hpf. Only injecting wild-type GLE1 mRNA, but not the FinMajor mutant form, rescues the head cell death. At least three independent injections were performed for each condition. Values are mean ± s.e.m. n, number of embryos analyzed in each condition. |
|
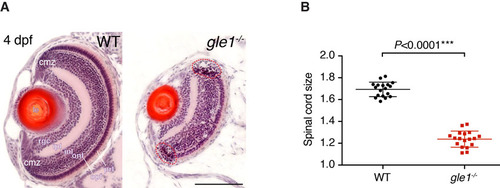
gle1-/- mutants have small eyes and spinal cord. (A) H&E-stained frontal transverse sections through the eyes of wild-type (WT) and gle1-/- mutant larvae at 4 dpf. All retinal cell layers are present in the gle1-/- eye, albeit in reduced numbers. Cells with condensed nuclei typical of apoptotic cells can be seen in the gle1-/- retina, especially in the ciliary marginal zone (red dashed outlines). cmz, ciliary marginal zone; prl, photoreceptor layer; opl, outer plexiform layer; onl, outer nuclear layer; inl, inner nuclear layer; ipl, inner plexiform layer; rgc, retinal ganglion cell layer; le, lens. Images were taken under the same magnification. Scale bar: 100 μm. (B) Quantification of spinal cord size (representative spinal cord sections shown in Fig. 2K,L) by measuring the area of 7-μm transverse plastic sections of the spinal cord at the region above the yolk extension using ImageJ. Data were collected from three randomly selected 5-dpf gle1-/- mutant larvae and three of their wild-type siblings; six sections per larva were measured. Statistical significance was determined using the unpaired t-test. The spinal cords of the gle1-/- mutant larvae were approximately 73% of wild-type size. PHENOTYPE:
|
|
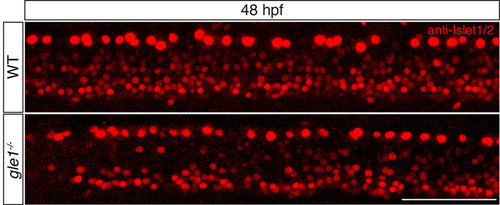
Anti-Islet1/2 staining of zebrafish at 48 hpf. An example of anti-Islet1/2 staining to reveal both the Rohon-Beard sensory neurons (which are the large cells close to the dorsal edge) and motoneurons (at the ventral edge of the spinal cord). Note that the gle1-/- mutant has fewer motoneurons than the wild type. See text and Table 1 for quantitative analysis. Images are lateral views of the trunk spinal cord with dorsal to top, anterior to left. Scale bar: 75 μm. |
|
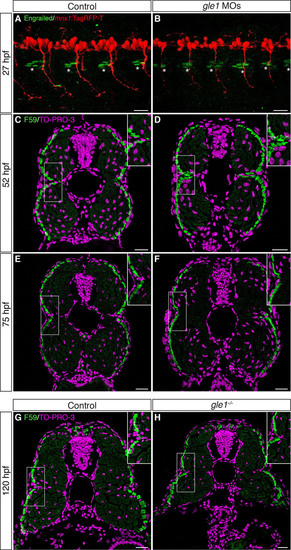
The integrity of the horizontal myoseptum in gle1 morphants and mutants appears intact. (A,B) Confocal images of control (A) and gle1 morphant (gle1 MOs) (B) zebrafish embryos with motoneurons labeled by mnx1:TagRFP-T transgene and muscle pioneers (MPs) labeled by anti-Engrailed/4D9 antibody (1:100 dilution) at 27 hpf. There were no discernible differences in the number and organization of the Engrailed+ MPs (asterisks) around the horizontal myoseptum between the control and gle1 morphant (<4-6 MPs per side in each somite segment). (C-H) Transverse cryosections through the trunk of controls (C,E,G), gle1 morphants (D,F) and gle1-/- mutant (H) were stained with anti-F59 antibody (1:100 dilution) to reveal the slow muscle fibers beneath the skin at 52 hpf (C,D), 75 hpf (E,F) and 120 hpf (G,H). At all three stages examined, the organization of the muscle fibers around the horizontal myoseptum (e.g. boxed areas, enlarged in insets) appeared indistinguishable between the controls and gle1 morphants/mutants. Nuclei were counterstained by TO-PRO-3. Scale bars: 25 μm. |
|
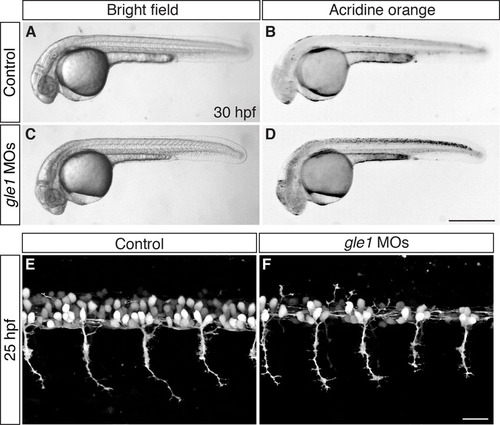
gle1 morphants have an earlier onset of phenotypes than gle1-/- mutants. (A-D) Embryos from gle1hi4161a/+ intercross were injected with a mix of two non-overlapping gle1 translation-blocking morpholinos (gle1 MOs, C,D) or with five-nucleotide-mismatched MOs (5mm gle1 MOs) (control, A,B). At 30 hpf, cell death can be seen in the CNS of the gle1 MO-injected morphants (bright field, C; Acridine Orange stained, D), but not in the embryos injected with the 5mm gle1 MOs (bright field, A; Acridine Orange stained, B). (E,F) Motoneuron development was examined by confocal microscopy in embryos from gle1hi4161a/+ intercross injected with either the gle1 MOs (F) or the 5mm gle1 MOs (E) as described in A-D. At 25 hpf, the gle1 MO-injected embryo (F) develops fewer motoneurons in the spinal cord than the 5mm gle1 MO-injected embryo (E). However, motor axon outgrowth in the gle1 MO-injected embryo still appears normal as compared with the control (E) at this stage. All images are lateral views of the spinal cord. Motoneurons (E,F) were imaged at somites 12-15 above the yolk extension with dorsal to top, anterior to left. Scale bars: 500 μm in D; 25 μm in F. |
|
TUNEL assays rarely detect motoneuron death in Gle1-deficient embryos. (A-L) Transverse cryosections through the spinal cords of the control (A-D), gle1-/- mutant (E-H) and gle1 morphant (I-L) embryos with their motoneuron nuclei labeled by mnx1:H2A-TagRFP-T transgene at 49 hpf. Sections were subjected to TUNEL reactions to reveal apoptotic cells. Nuclei were counterstained by TO-PRO-3. Note that motoneurons (H2A-TagRFP-T+) are rarely labeled by TUNEL (<1%, examples indicated by arrows) in the gle1-/- mutant (E-H) and gle1 morphant (I-L) embryos, despite the fact that TUNEL+ signals are evident in those sections with deficient Gle1 activity (E-L). Scale bar: 10 μm. |
|
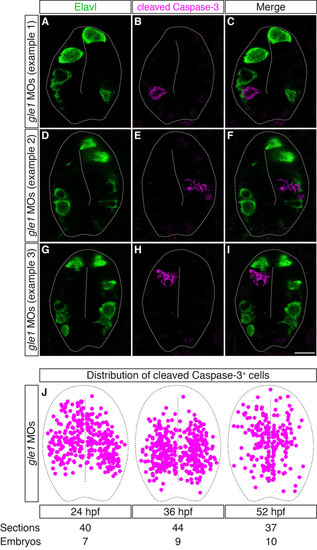
The distribution of cleaved caspase 3+ cells is mutually exclusive with that of differentiated neurons in Gle1-deficient embryos. (A-I) Transverse cryosections through the spinal cords of gle1 morphants were co-stained with anti-Elavl and anti-cleaved caspase 3 antibodies at 24 hpf. The cleaved caspase 3+ cells (B,E,H) are distributed widely along the dorsoventral axis of the spinal cord, often located close to the central canal or immediately adjacent to the basally located Elavl+ neurons (A,D,G). However, cleaved caspase 3+ and Elavl+ signals do not overlap (merged channels, C,F,I). Each image is a single optical section (<1 µm) extracted from the confocal z-stack with dorsal upward. Dashed lines outline spinal cords and central canals. Scale bar: 10 µm. (J) Distribution of the cleaved caspase 3+ cells in the transverse sections of the gle1 morphant spinal cords at 24, 36 and 52 hpf. Single confocal sections were manually superimposed and the positions of the cleaved caspase 3+ cells were marked. The cleaved caspase 3+ cells are distributed widely along both the dorsoventral and apical-basal axes of the spinal cord. |
|
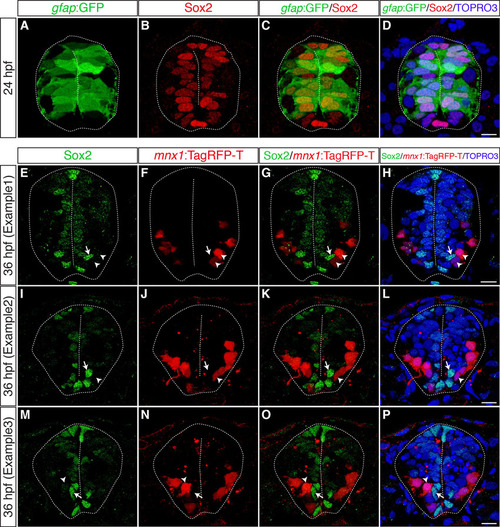
Most gfap:GFP+ radial glia are Sox2+ neural precursors, which are located close to the apical surface and are mutually exclusive with motoneurons. (A-D) Transverse cryosections through the spinal cord of wild-type embryos harboring the gfap:GFP transgene were stained with anti-Sox2 antibody at 24 hpf. Most, if not all, gfap:GFP+ cells (A) are also Sox2+ (B, merged channels in C,D). (E-P) Transverse cryosections through the spinal cord of gle1 morphants harboring the mnx1:TagRFP-T transgene were stained with anti-Sox2 antibody at 36 hpf. Note that a portion of mnx1:TagRFP-T+ motoneurons (arrowheads) are located in close proximity to some of the Sox2+ neural precursors (arrows). However, these two signals do not overlap (merged channels in G,K,O and H,L,P). Nuclei were counterstained by TO-PRO-3. Scale bars: 7.5 μm in D; 10 μm in H,L,P. |
|
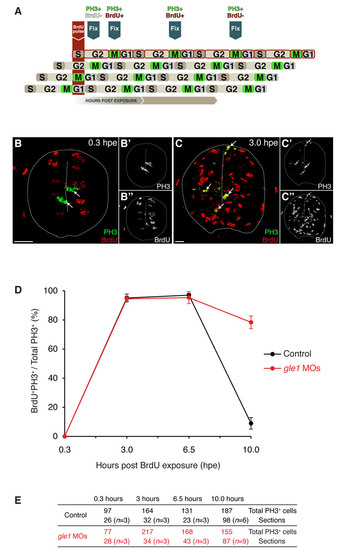
Spinal neural precursors fail to exit the cell cycle efficiently upon Gle1 depletion. (A) Schematic of the ′percent labeled mitoses′ (PLM) paradigm. A 45-minute BrdU pulse marks S-phase neural precursors, which then progress through the cell cycle over time. Detection of M-phase cells with anti-phosphohistone H3 (PH3) antibody identifies cells that were in S phase (PH3+ BrdU+) or not in S phase (PH3+ BrdU-) at the time of BrdU exposure. (B-C22) Examples of transverse spinal cord sections showing PH3 staining (green) and BrdU incorporation (red) at 0.3 (B) and 3.0 (C) hours post-exposure (hpe). All M-phase cells were negative for BrdU labeling at 0.3 hpe (arrows in B). However, many S-phase cells have progressed to M phase within 3 hours and were detected by PH3/BrdU co-labeling (arrows in C). Single-channel images of PH3 (B2,C2) and BrdU (B22,C22) are shown on the right. Scale bars: 10 µm. (D,E) Plotting of PLM from 0.3 to 10 hpe (D). The embryos were at 26 hpf at the time of BrdU pulse. Before 32.5 hpf (6.5 hpe), the neural precursors progressed through the cell cycle at a similar rate between the controls and gle1 morphants (<3.5 hours for each full cell cycle). However, after 32.5 hpf, the cell cycle progression of the neural precursors in the controls has slowed down: after 3.5 hours (i.e. at the 10.0 hpe time point), fewer than 10% of cells reached M phase. By contrast, <80% of the neural precursors in the gle1 morphants still progressed through the cell cycle and reached the next M phase at 10.0 hpe. These results indicate that Gle1-deficient neural precursors fail to exit the cell cycle efficiently. Co-labeling of PH3/BrdU was examined manually in single confocal optical sections (< 1 μm). Values are graphed as mean ± s.e.m. (E) Quantification of the sections containing PH3+ cells at different time points after BrdU exposure. n, number of embryos analyzed at each time point. |
|
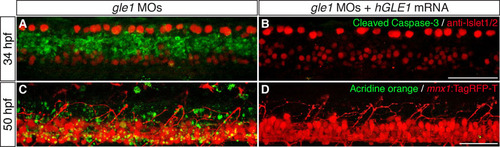
Cell death in the spinal cords of gle1 morphants is suppressed by expressing human GLE1. (A,B) gle1 morphants injected with buffer alone (A) or with <140 pg of in vitro transcribed EGFP-tagged human GLE1b mRNA (B) were co-stained with anti-Islet1/2 and anti-cleaved caspase 3 antibodies at 34 hpf. Note that the cleaved caspase 3 signals were diminished when human GLE1 was expressed in the gle1 morphant (B). (C,D) gle1 morphants with their motoneurons labeled by mnx1:TagRFP-T transgene were injected with buffer alone (C) or with <140 pg of in vitro transcribed EGFP-tagged human GLE1b mRNA (D). These morphants were stained with Acridine Orange to reveal dying cells and the live embryos were imaged at 50 hpf. Note that apoptotic cell death was suppressed by expressing human GLE1 (D). Images are lateral views of the trunk spinal cord with dorsal to top, anterior to left. Scale bars: 50 μm. |
|
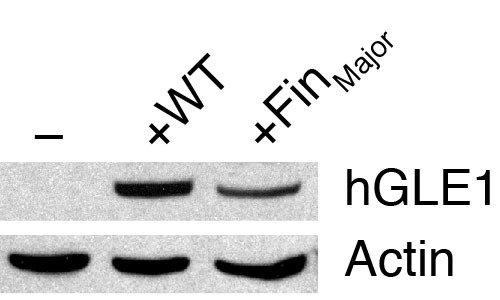
Western blot analyses of human GLE1 expression in zebrafish embryos. gle1 morphants injected with buffer alone (-), wild-type human GLE1b mRNA (+WT), or human GLE1b_FinMajor mutant mRNA (+FinMajor) were lysed at the bud stage and subjected to western blot analyses using the anti-human GLE1 antibody (which does not recognize the zebrafish Gle1 protein). Anti-actin staining was also performed as a loading control. Note that both the wild-type and the FinMajor form of human GLE1 proteins were expressed in zebrafish embryos. |

Unillustrated author statements PHENOTYPE:
|