- Title
-
Hedgehog signaling via angiopoietin1 is required for developmental vascular stability
- Authors
- Lamont, R.E., Vu, W., Carter, A.D., Serluca, F.C., MacRae, C.A., and Childs, S.J.
- Source
- Full text @ Mech. Dev.
|
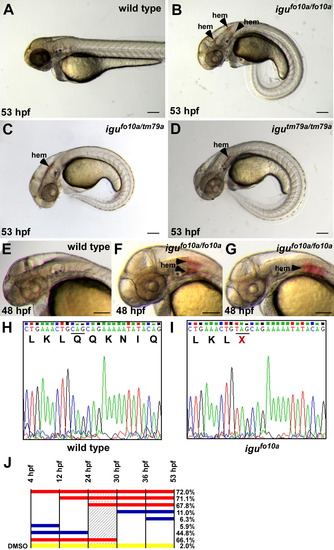
Loss of hedgehog signaling leads to vascular stability defects. (A–D) igufo10a mutants present with a curled body axis and hemorrhage (hem) at 53 hpf (B), as compared to wild type siblings (A). igutm79a/igufo10a compound heterozygous embryos (C) and igutm79a mutant embryos (D) both present with hemorrhage at 53 hpf. (E–G) While wild type siblings do not show any signs of hemorrhage at 48 hpf (E), hemorrhages are present in the head of igufo10a mutants (F and G). (H and I) Electropherograms of dzip1 sequence from wild type (H) and igufo10a mutants (I) showing the presence of a Q339X mutation. (J) Hemorrhage rate after treatment with cyclopamine for developmental windows. Solid bars indicate the treatment interval with the percent of embryos displaying hemorrhage at 53 hpf given on the right side. Red bars indicate a treatment interval that results in maximal hemorrhage rates, while blue bars indicate intervals that produce minimal hemorrhage. The yellow bar indicates DMSO controls treated for the entire interval. The shaded area represents the most sensitive interval to cyclopamine treatment. Scale bar in (A)–(G) is 500 μm. PHENOTYPE:
|
|
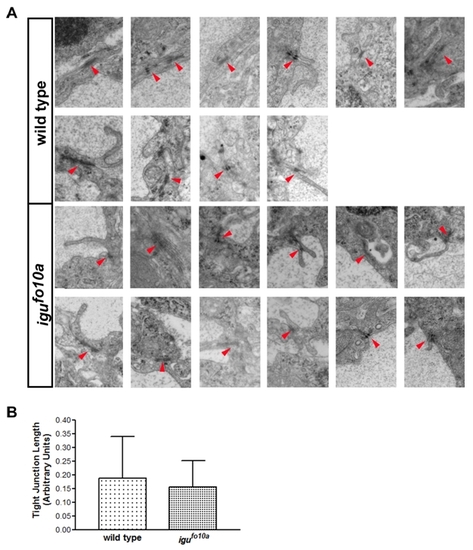
igufo10a mutants have cell attachment and vessel caliber defects at 48 hpf. (A–D) TEM images of a single dorsal aortae in the head region of wild type (A and C) and igufo10a mutant embryos (B and D) at 48 hpf. In wild type embryos (A), the dorsal aorta is lateral to the notochord (nc) and is comprised of endothelial cells (ec) and a lumen (lum) tightly surrounded by perivascular support cells (*). Conversely, in igufo10a mutants (B) the perivascular cells make few contacts with endothelial cells. Tight junctions (arrows) are readily identified in both wild type (C) and igufo10a mutants (D). Boxes in (A) and (B) show area of magnification in (C) and (D). (E and F) Confocal Z-stack projections of wild type (E) and igufo10a mutants (F) at 48 hpf reveals that the size of the dorsal aortae (indicated by arrow) in igufo10a mutants is significantly smaller than that found in wild type. Red lines in (E) and (F) indicate plane of sectioning in (A)–(D). Scale bar in (A) and (B) is 2 μm, (C) and (D) is 300 nm, and (E) and (F) is 100 μm. PHENOTYPE:
|
|
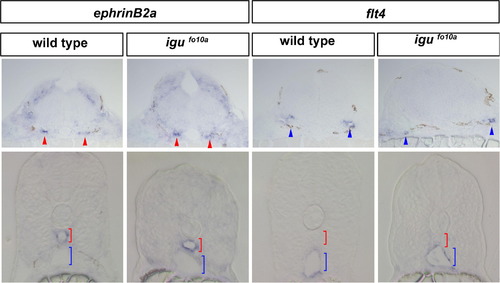
Artery and vein marker expression is normal in igufo10a mutants Indistinguishable expression of the artery marker efnb2a is observed in cross-sections of the lateral dorsal aortae (red arrows) of the hindbrain and the dorsal aorta (red bracket) of the trunk in both wild type and igufo10a mutants at 28 hpf. Indistinguishable expression of the venous marker flt4 is observed in cross-sections of veins in the hindbrain (blue arrows) and posterior cardinal vein of the trunk (blue bracket) at 28 hpf. Scale bar is 30 μm. EXPRESSION / LABELING:
|
|
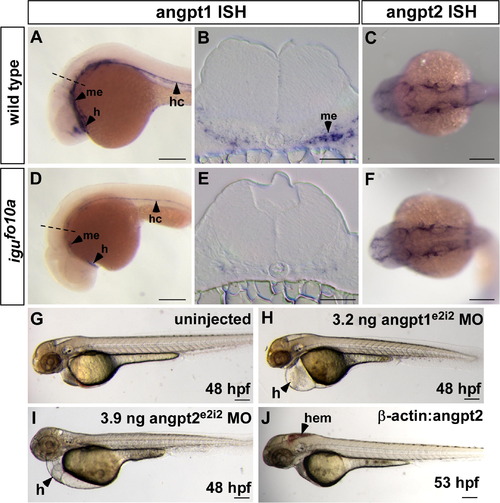
Loss of Angiopoietin1–Tie signaling in igufo10a mutants results in hemorrhage. (A–F) (A and B) In wild type embryos at 28 hpf, angpt1 is expressed in the developing heart (h), hypochord (hc), and ventral mesenchyme (me) as shown in whole mount (A) and in cross-section (B). (D and E) In igufo10a mutants of the same age, angpt1 expression persists in the heart and hypochord, but is substantially down-regulated in ventral mesenchyme, as shown in whole mount (D) and in cross-section (E). The dashed line in (A) and (D) marks the level of the section in (B) and (E), respectively. (C and F) Expression of angpt2 is unchanged in igufo10a mutant embryos (F) compared to wild type sibs (C) at 28 hpf. (G–I) Injection of 3.2 ng of angpt1e2i2 MO (H) or 3.9 ng of angpt2e2i2 MO (I) into wild type embryos results in defective heart development, pericardial edema (H), and lack of circulation when compared to uninjected control embryos at 48 hpf (G). (J) Transgenic over-expression of angpt2 under the β-actin promoter results in hemorrhage (hem) in wild type embryos at 53 hpf. Scale bar is 500 μm in (A), (C), (D), and (F)–(J) and 50 μm in (B) and (E). |
|
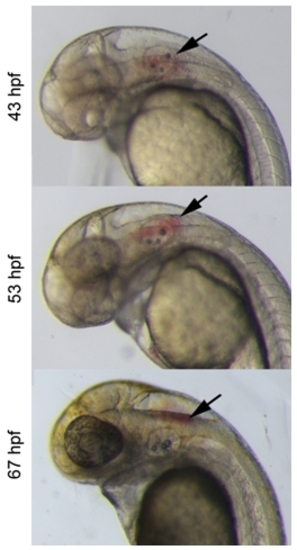
Multiple vessel origins for hemorrhages in igufo10 mutants at 48- 52 hpf |
|
Blood moves dorsally after hemorrhage in the head |
|
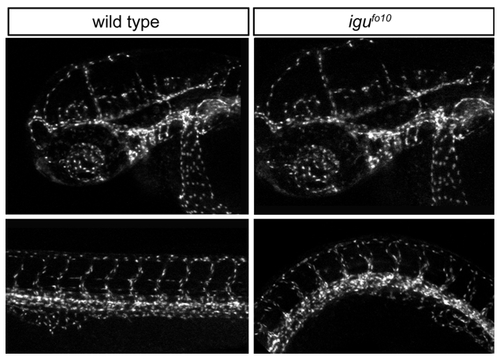
Endothelial tight junctions are formed normally in igufo10 mutants |
|
Vessel patterning in the head and trunk of igu embryos appears normal |
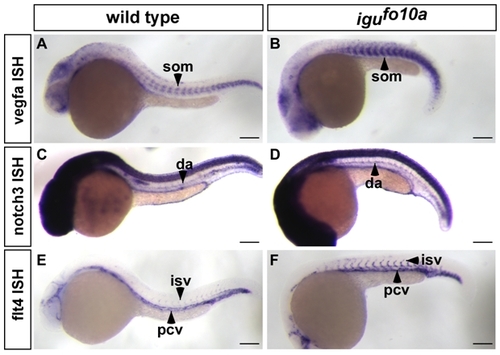
|
Artery-vein identity is normal in igufo10a mutants at 28 hpf. (A-B) vegfa is unchanged in the ventral head and somites (som) in both wild type (A) and igufo10a mutants (B). (C-D) notch3 expression in the dorsal aorta (da) is unchanged in both wild type (C) and igufo10a mutants (D). (E-F) flt4 expression is restricted to the posterior cardinal vein (pcv) and intersegmental vessels (isv) in both wild type (E) and igufo10a mutants (F). Scale bar is 600 μm. |
|
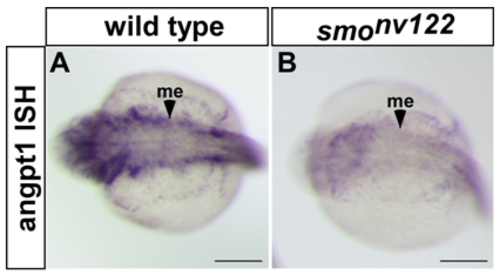
angpt1 is dramatically downregulated in smonv122 mutants. (A, B) angpt1 is strongly expressed in ventral mesenchyme (me) in wild type embryos (A) at 28 hpf, but is almost absent in smonv122 mutants (B) at the same age. Scale bars are 500 μm. |
|
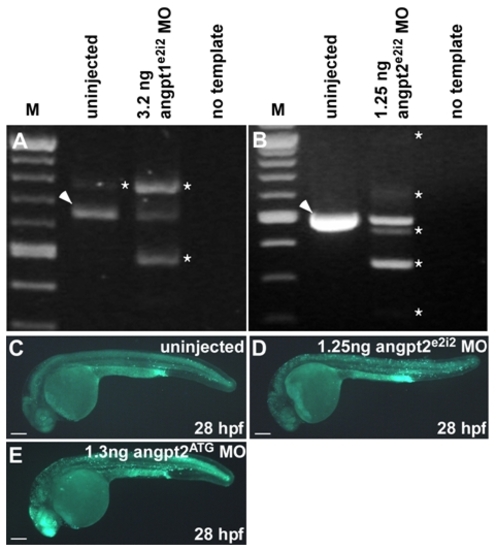
Morpholino efficiency and off-target cell death measurements in angpt1e2i2 and angpt2e2i2 morphants. (A) RT-PCR of a fragment surrounding the exon 2-intron 2 boundary from angpt1e2i2 morphants injected with 3.2 ng of angpt1e2i2 MO showing a shift in size from the predominant isoform (arrowhead) to that of higher and lower molecular weights (asterisk) in comparison to uninjected control embryos. (B) RT-PCR of a fragment surrounding the exon 2-intron 2 boundary from angpt2e2i2 morphants injected with 1.25 ng angpt2e2i2 MO (the dose used to rescue hemorrhage in igufo10a mutants). Morpholino injection results in a shift from the wild type band in uninjected controls (arrowhead) to the presence of five additional bands of differing sizes representing different mis-spliced transcripts (asterisk) and reduced presence of a wild type sized band. (C-E) Off-target morpholino-induced cell death assessment using acridine orange. Epifluorescent images of uninjected embryos (C) incubated in acridine orange displaying basal levels of apoptosis. Angpt2e2i2 (D) and angpt2ATG (E) morphants injected with 1.25 ng or 1.3 ng of morpholino, respectively, display increased levels of apoptosis in the head region. This increase in apoptosis does not likely influence our rescue results since we would expect increased neural death to exacerbate hemorrhage, not rescue it. In A and B, “M” denotes New England Biolabs 100bp ladder. Scale bars in C-E are 500 μm. |
Reprinted from Mechanisms of Development, 127(3-4), Lamont, R.E., Vu, W., Carter, A.D., Serluca, F.C., MacRae, C.A., and Childs, S.J., Hedgehog signaling via angiopoietin1 is required for developmental vascular stability, 159-168, Copyright (2010) with permission from Elsevier. Full text @ Mech. Dev.