- Title
-
RBP4 Disrupts Vitamin A Uptake Homeostasis in a STRA6-Deficient Animal Model for Matthew-Wood Syndrome
- Authors
- Isken, A., Golczak, M., Oberhauser, V., Hunzelmann, S., Driever, W., Imanishi, Y., Palczewski, K., and von Lintig, J.
- Source
- Full text @ Cell Metab.
|
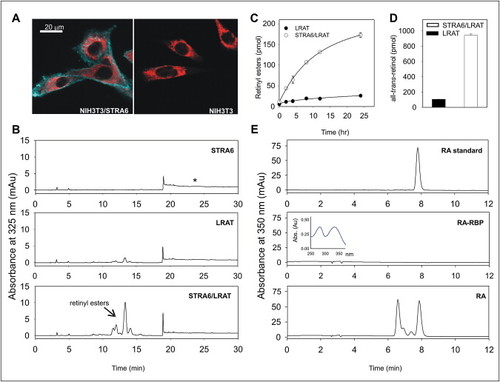
STRA6-Dependent Uptake and Release of Retinoids |
|
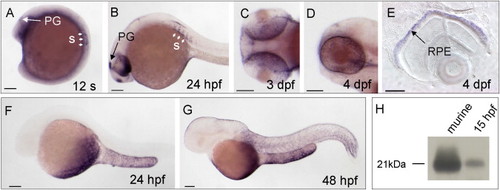
stra6 and rbp4 mRNA Expression during Zebrafish Development Analyzed by Whole-Mount In Situ Hybridization EXPRESSION / LABELING:
|
|
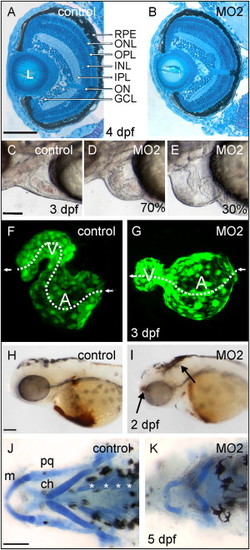
Targeted Gene Knockdown of Stra6 Causes Embryonic Abnormalities and Vitamin A Deficiency in Developing Eyes PHENOTYPE:
|
|
Stra6 Deficiency Causes Multisystemic Malformations in Zebrafish |
|
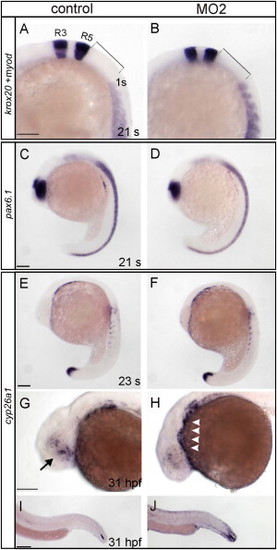
Comparison of Marker Gene Expression between Control and stra6 Morphant Embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
|
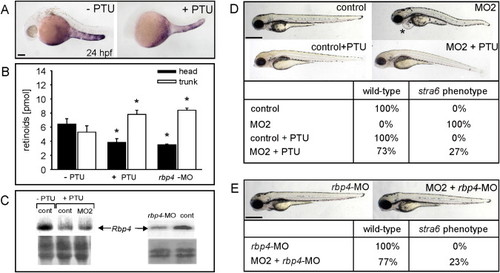
PTU and rbp4-MO Treatments Prevent Developmental Abnormalities in stra6 Morphants PHENOTYPE:
|
Reprinted from Cell Metabolism, 7(3), Isken, A., Golczak, M., Oberhauser, V., Hunzelmann, S., Driever, W., Imanishi, Y., Palczewski, K., and von Lintig, J., RBP4 Disrupts Vitamin A Uptake Homeostasis in a STRA6-Deficient Animal Model for Matthew-Wood Syndrome, 258-268, Copyright (2008) with permission from Elsevier. Full text @ Cell Metab.