- Title
-
The zebrafish prospero homolog prox1 is required for mechanosensory hair cell differentiation and functionality in the lateral line
- Authors
- Pistocchi, A., Feijoo, C.G., Cabrera, P., Villablanca, E.J., Allende, M.L., and Cotelli, F.
- Source
- Full text @ BMC Dev. Biol.
|
Prox1 expression in the lateral line system of zebrafish embryos. (A) In situ hybridization of prox1 at 30 hpf shows expression in the CNS and in the lateral line migrating primordium (box). (B) Enlarged view of a prox1 positive deposited neuromast in the posterior lateral line at 30 hpf. (C, E) Immunofluorescence using an anti-Prox1 antibody at 48 hpf and 96 hpf, arrows indicate the deposited neuromasts. (D, F) Close up of Prox1 expression in a neuromast at the two stages examined. (G, H, I) Immunofluorescence labeling in neuromasts with anti GFP (G), anti Prox1 (H) and the cell nuclei with DAPI (I) in 96 hpf pou4f1::GFP transgenic larvae. Scale bar = 10 micron. |
|
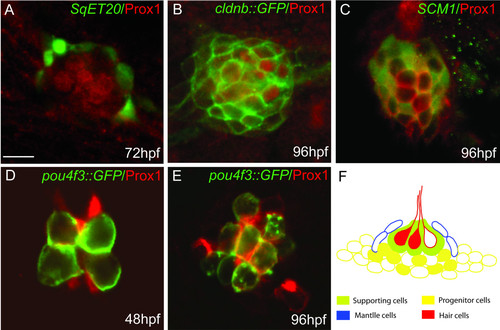
Co-localization of Prox1 protein with different cell type markers in the lateral line system. Immunofluorescence showing Prox1 (red) in different GFP transgenic lines (green) at 48, 72 and 96 hpf: (A) SqET20 that labels mantle cells; (B) cldnb::GFP that labels all cells that form the neuromast; (C) SCM1 that labels presumptive progenitor cells; (D, E) pou4f3::GFP that labels hair cells. (F) Schematic representation of a neuromast showing the different cell types. Prox1 expression is represented as filled cells and can be seen among hair cells (red) and underlying supporting cells (green) and/or progenitor cells (yellow). Prox1 is absent from mantle cells (blue). Scale bar = 5 micron. |
|
Diasp staining in control and prox1 loss of function embryos at 48 hpf. (A, B) Microinjection of prox1 MO decreases the number of Diasp positive cells in comparison to control embryos at the same developmental stage. (C, D) The number of Diasp-labeled neuromasts per larva at 48 hpf were counted and larvae were classified according to the number of neuromasts present on one side. While most control larvae show between 5 and 8 neuromasts (C), prox1 MO injected larvae display between 0 and 5 neuromasts per side (D). PHENOTYPE:
|
|
prox1 loss of function of does not affect PLL primordium cell number or PLL nerve development. (A, B) GFP labeled primordia migrating at 32 hpf in cldnb::GFP transgenic fish. The size of the primordium is not affected in morphant embryos in comparison to control embryos at the same developmental stage. (C, D, C′, D′) Acetylated tubulin immunostaining indicates that the lateral line nerve is not perturbed in morphant embryos (white arrows) while differentiated hair cell with their kinocilia (brown arrows C, D and in fluorescence at higher magnification C′, D′), are absent in morphant fish in comparison to control embryos. (E, F) prox1 MO injected embryos do not show increased cell death as indicated by the TUNEL assay, in comparison to control embryos. n, notochord; y, yolk. Scale bar = 15 micron in A, B, C, D, E, F and 3 micron in C′, D′. |
|
prox1 loss of function affects development of functional hair cells in zebrafish lateral line neuromasts. Comparison of GFP expression between morphant and control embryos at 60 hpf shows no differences in mantle cells (A, B), progenitor cells (C, D) and hair cells (E, F) in different transgenic backgrounds, indicating that there is no effect of the prox1 morpholino in the specification of these cell types in the neuromast. (G, H) However, the vital stain Diasp indicates that almost 50% of the hair cells present in a neuromast are not functionally active. (I) Comparison of the percentage of GFP labeled hair cells that are co-labeled with Diasp in control and prox1 MO injected embryos. Scale bar = 10 micron. |
|
Decreased levels of Prox1 protein in prox1 loss of function embryos. Immunohistochemistry using an anti-Prox1 antibody at 48 hpf (A) Prox1 protein distribution in control embryos in comparison to prox1 MO injected embryos (B) black arrow lens (l); arrowhead diencephalon (d), brown arrow diencephalic-mesencephalic boundary (dmb). Scale bar = 200 micron. EXPRESSION / LABELING:
PHENOTYPE:
|
|
DiAsp staining in control and prox1 loss of function embryos at 72 hpf. As at 48 hpf, also at 60 and 72 hpf, prox1 MO injected embryos still presented a decrease number of DiAsp positive cells in neuromasts in comparison to control embryos at the same developmental stage, indicating that the effect is not due to developmental delay of morphant embryos. |