- Title
-
Photodynamic Therapy Using a Heavy-Atom-Free G‑Quadruplex-Targeted Photosensitizer to Efficiently Regress Rhabdomyosarcoma Tumors In Vivo
- Authors
- Rodriguez-Marquez, E., Nord, H., Puchán Sánchez, D., Kassem, A., Andrés Castán, J.M., Deiana, M., Cabanetos, C., Sabouri, N., von Hofsten, J.
- Source
- Full text @ ACS Pharmacol Transl Sci
|
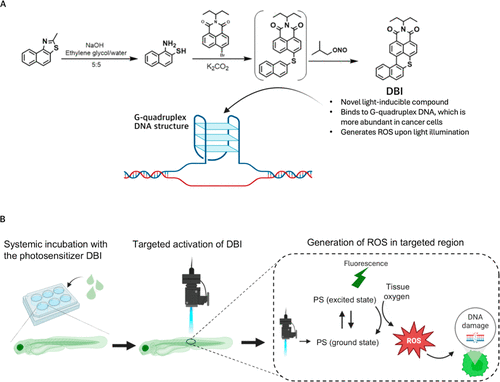
DBI-mediated PDT. (A) Synthesis of DBI. (B) Schematic illustration of PDT using DBI. Zebrafish are systemically incubated with DBI, which is subsequently activated in the target region. Upon activation, DBI releases fluorescence and generates ROS, leading to DNA damage and apoptosis. |
|
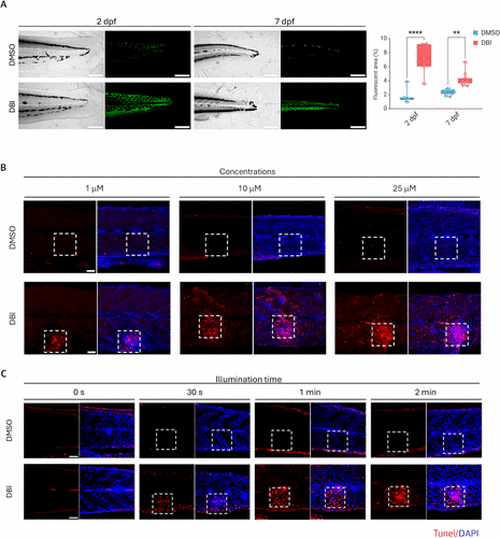
Uptake and activation of DBI in zebrafish. (A) Confocal laser scanning microscopy images of 2 days postfertilization (dpf) and 7 dpf AB zebrafish larvae treated with DMSO (control) or DBI, followed by illumination. Grayscale images (left) and corresponding green fluorescent signals (right) are shown. Scale bar = 200 μm. p < 0.01 (**), p < 0.0001 (****). (B,C) Apoptosis detection in zebrafish after DMSO or DBI treatment, followed by illumination, using different DBI concentrations: 1,10 and 25 μM (B) and different light exposure durations: 0 s to 2 min (C). Dashed squares indicate the illuminated areas. Apoptotic cells were stained with TUNEL (red), and nuclei were counterstained with DAPI (blue). Scale bar = 50 μm. |
|
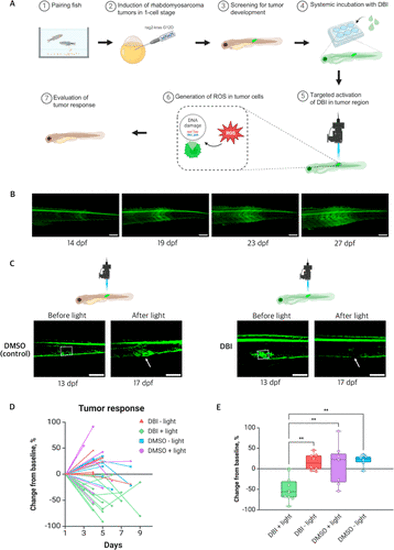
Effects of PDT on kRAS-induced RMS tumors in pax3a:EGFP zebrafish. (A) Schematic illustration of the induction of RMS tumors in zebrafish, followed by treatment with DBI and evaluation of the tumor response. (B) Representative image of RMS tumor progression in pax3a:EGFP zebrafish line, from 14 to 27 days post fertilization (dpf). The tumor region is identified by high levels of GFP expression. Scale bar = 200 μm. (C) Confocal images of tumors before and after treatment with DMSO + light (left) and DBI + light (right). Dashed squares indicate the illuminated areas. Tumor responses are indicated with the white arrows. Scale bar = 200 μm. (D) Comparison of tumor area, expressed as a percentage of initial size, after treatment across four conditions: DBI + light (treatment), and three controls: DBI – light, DMSO + light, and DMSO – light. (E) Percentage change in tumor area at 4–5 days post-treatment across the four conditions. p < 0.01 (**). |
|
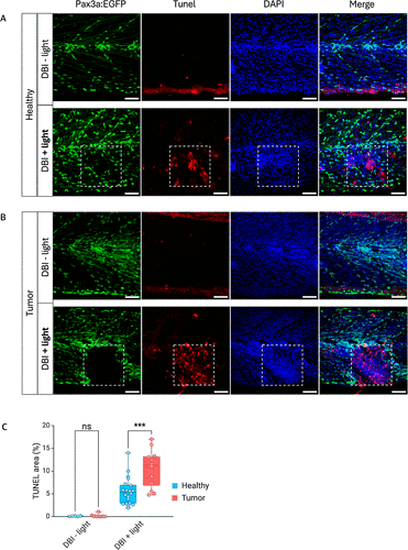
Apoptosis detection in zebrafish after DBI treatment. (A) Representative images of 2 week-old pax3a:EGFP zebrafish without tumors treated with DBI, followed or not by illumination. (B) Sibling zebrafish with rhabdomyosarcoma tumors treated with DBI, followed or not by illumination. Dashed squares indicate the illuminated areas. Apoptotic cells were detected with TUNEL staining (red), and nuclei were counterstained with DAPI (blue). Scale bar = 50 μm. (C) Quantification of TUNEL-stained area in healthy and tumor regions, treated with DBI, followed or not by illumination. p < 0.001(***). |
|
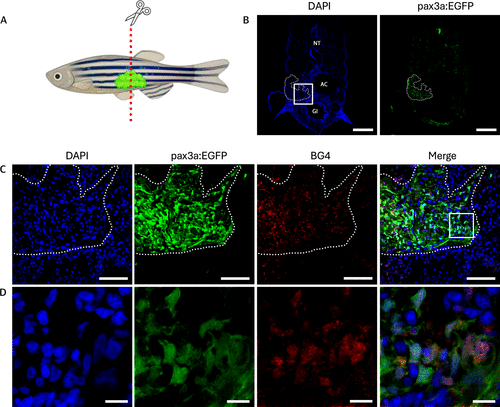
Elevated levels of G4 structures in RMS tissue sections of pax3a:EGFP zebrafish. (A) Schematic illustration of 2 month-old pax3a:EGFP zebrafish with RMS tumor. (B) Tissue section of the tumor, showing DAPI (left) and pax3a:EGFP (right). NT: neural tube, AC: abdominal cavity, GI: gastrointestinal tract. Dashed lines indicates where the tumor is located. Scale bar = 200 μm. (C) Zoom of the boxed region from (B), showing DAPI in blue, pax3a:EGFP expression in green, BG4 staining for G4 DNA in red, and a merged image of all channels. Scale bar = 50 μm. (D) Zoom of the boxed region from (C). Scale bar = 10 μm. |
|
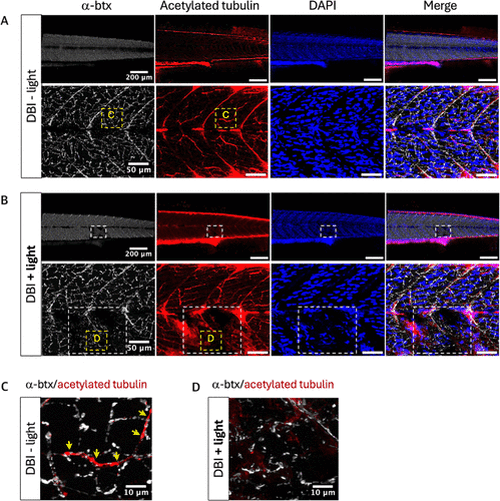
Effects of DBI on neuromuscular junctions. (A) Zebrafish treated with DBI without light. (B) Zebrafish treated with DBI and light. White dashed squares indicate the illuminated areas. (C) Zoom of a nonilluminated region from Figure 4A, taken from the yellow dashed square region. The yellow arrows indicate the overlap of α-btx and acetylated tubulin. (D) Zoom of the illuminated region from Figure 4B, taken from the yellow dashed square region. Neuromuscular junctions are shown by α-btx staining (white) and axonic microtubules are shown by acetylated tubulin staining (red). Nuclei were counterstained with DAPI (blue). |