- Title
-
Lurasidone induces developmental toxicity and behavioral impairments in zebrafish embryos
- Authors
- Li, W., Wang, F., Feng, Z., Cheng, Q., Huang, Y., Zhu, L., Xiao, H., Gong, H.
- Source
- Full text @ Front Psychiatry
|
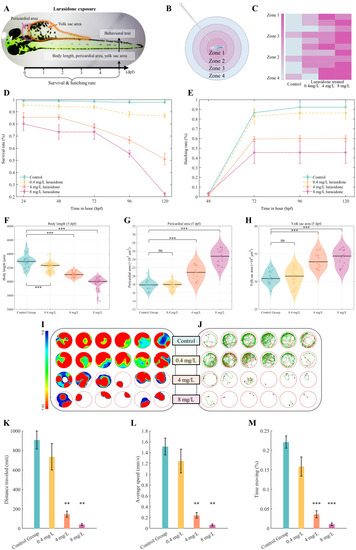
Developmental toxicity assessment of lurasidone exposure on zebrafish embryos. |
|
Lurasidone induces dose-dependent neuronal apoptosis in zebrafish embryos. |
|
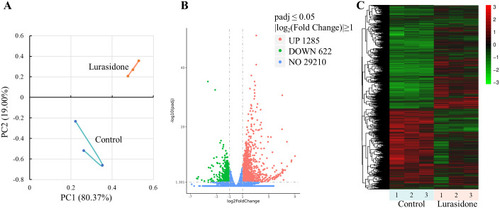
Transcriptome analysis of zebrafish embryos exposed to Lurasidone. |
|
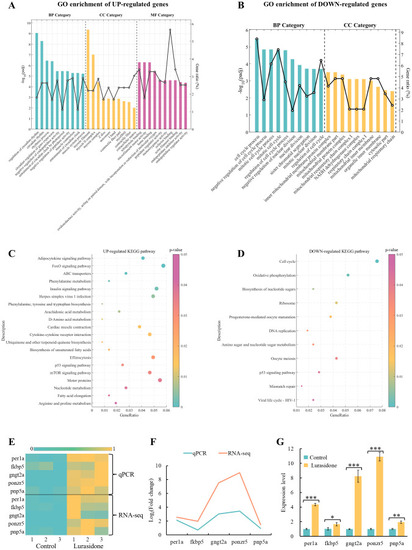
GO enrichment and KEGG pathway analysis of differentially expressed genes (DEGs), and transcriptional and qPCR validation of key genes in Lurasidone-treated zebrafish embryos. |
|
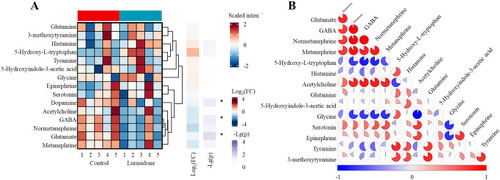
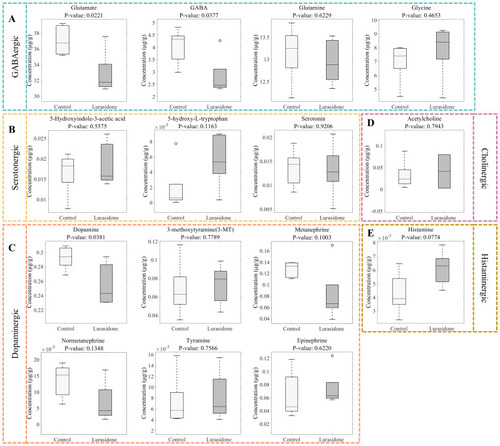
Systematic alterations of 15 neurotransmitters in zebrafish larvae following lurasidone exposure. |
|
Quantitative analysis of neurotransmitter alterations in zebrafish larvae following lurasidone exposure. |