- Title
-
Activity-driven myelin sheath growth is mediated by mGluR5
- Authors
- Braaker, P.N., Mi, X., Soong, D., Bin, J.M., Marshall-Phelps, K., Bradley, S., Benito-Kwiecinski, S., Meng, J., Arafa, D., Richmond, C., Keatinge, M., Yu, G., Almeida, R.G., Lyons, D.A.
- Source
- Full text @ Nat. Neurosci.
|
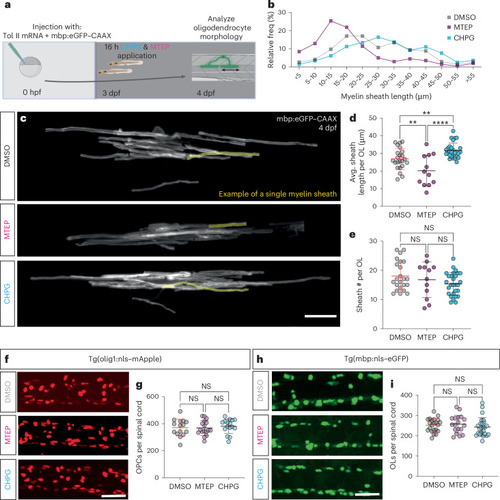
mGluR5 stimulation increases myelin sheath length without affecting cell number. |
|
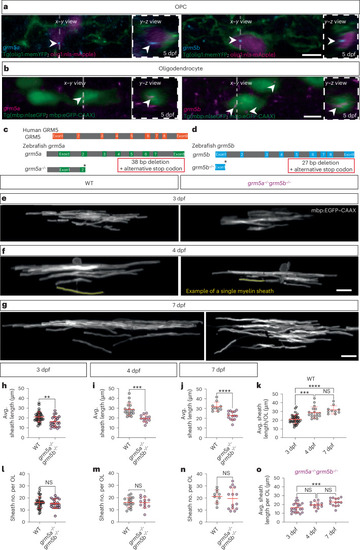
Mutations in genes encoding mGluR5 reduce myelin sheath length without affecting sheath number. |
|
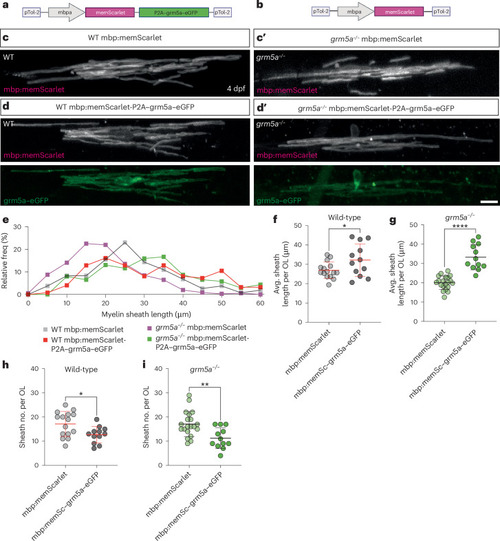
Oligodendrocyte-restricted expression of |
|
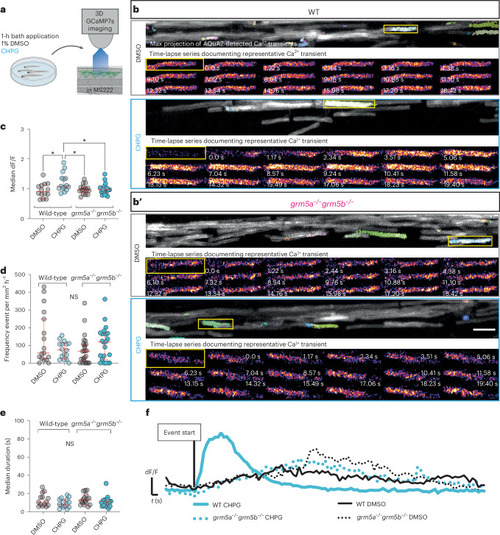
Endogenous mGluR5 is required for CHPG-induced high-amplitude myelin Ca2+ transients to occur. |
|
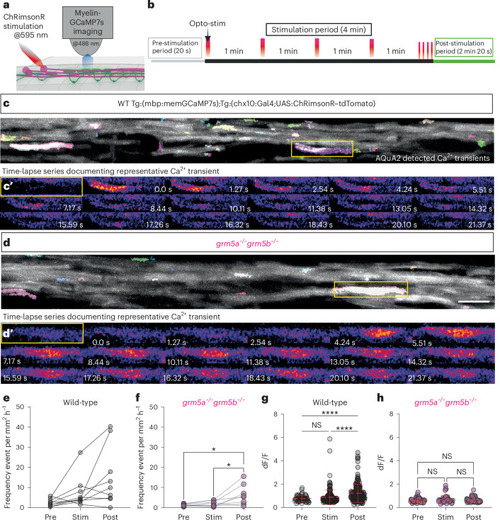
Opto-stimulation of neuronal activity induces mGluR5-dependent high-amplitude myelin Ca2+ transients. |
|
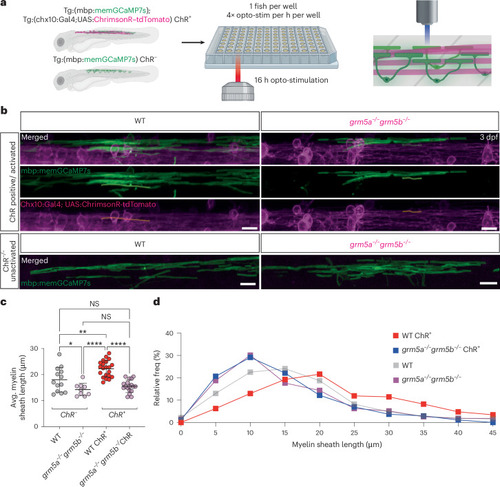
mGluR5 mediates myelin sheath growth in response to opto-stimulation of |