- Title
-
Role of the TPR family protein VPA1365 in regulating type III secretion system 2 and virulence in Vibrio parahaemolyticus
- Authors
- Yin, W., Wan, M., Zhang, Y., Meng, H., Pan, Z., Jiao, X., Gu, D.
- Source
- Full text @ Appl. Environ. Microbiol.
|
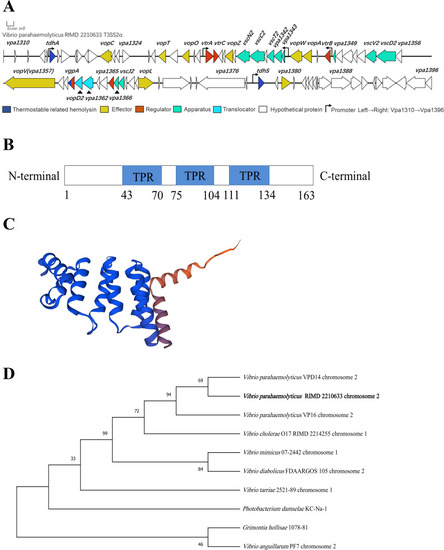
Conserved domain structure and phylogenetic tree analysis of VPA1365 in |
|
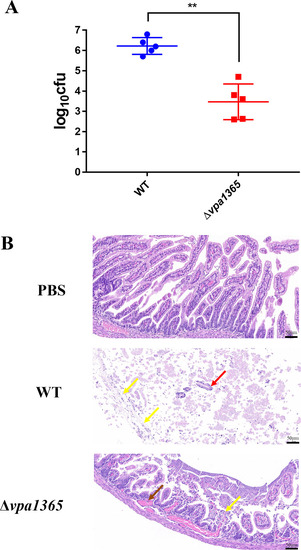
Analysis of the intestinal pathological section and bacterial colonization quantity in infant rabbits. ( |
|
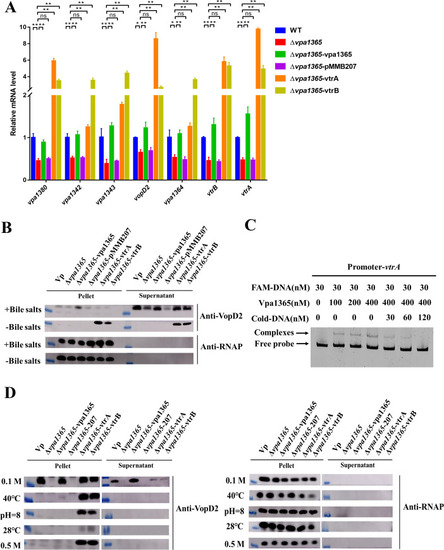
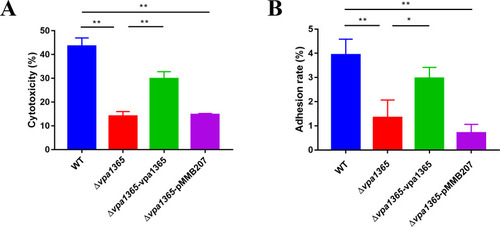
The effects of |
|
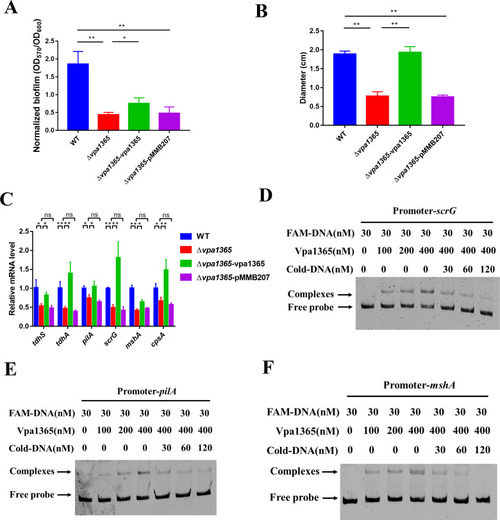
The effects of |
|
The effects of |
|
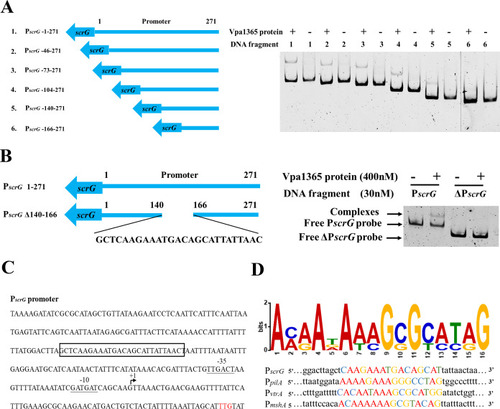
Identification of the VPA1365-binding motifs. ( |
|
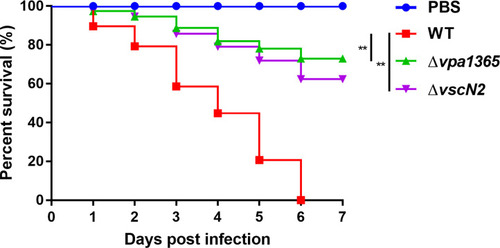
The survival rate of zebrafish challenged with WT, Δ |
|
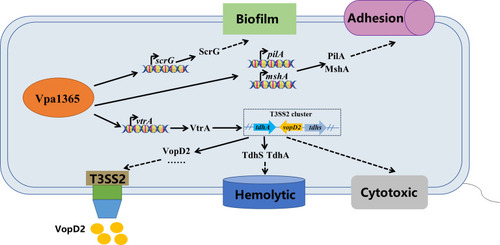
The putative model of VPA1365 regulation in |